

非共轭丁烯内酯α-和β-位反应研究进展
收稿日期: 2022-10-28
修回日期: 2023-01-16
网络出版日期: 2023-02-07
基金资助
国家自然科学基金(22001219); 四川省自然科学基金(2022NSFSC1189)
Advances on the α- and β-Reactions of Deconjugated Butenolides
Received date: 2022-10-28
Revised date: 2023-01-16
Online published: 2023-02-07
Supported by
National Natural Science Foundation of China(22001219); Natural Science Foundation of Sichuan Province(2022NSFSC1189)
周林 , 杨鸿 , 杨川 , 赵志刚 , 李清寒 . 非共轭丁烯内酯α-和β-位反应研究进展[J]. 有机化学, 2023 , 43(7) : 2407 -2424 . DOI: 10.6023/cjoc202210035
Developing the construction methods of γ-lactone derivatives due to their anti-inflammatory, antitumor, anti-cancer and other biological activities has important value in organic synthesis. Among them, the deconjugated butenolides have shown great potential and advantages in the synthesis of γ-lactone derivatives. As the direct and efficient method, it has attracted more attentions of chemists, and is one of the hot topics of organic synthesis in recent years. The α- and β-reactions of deconjugated butenolides are reviewed, in which the characteristics of various reaction systems and the mechanisms of some reactions are discussed. At the same time, the future development is prospected in this field.
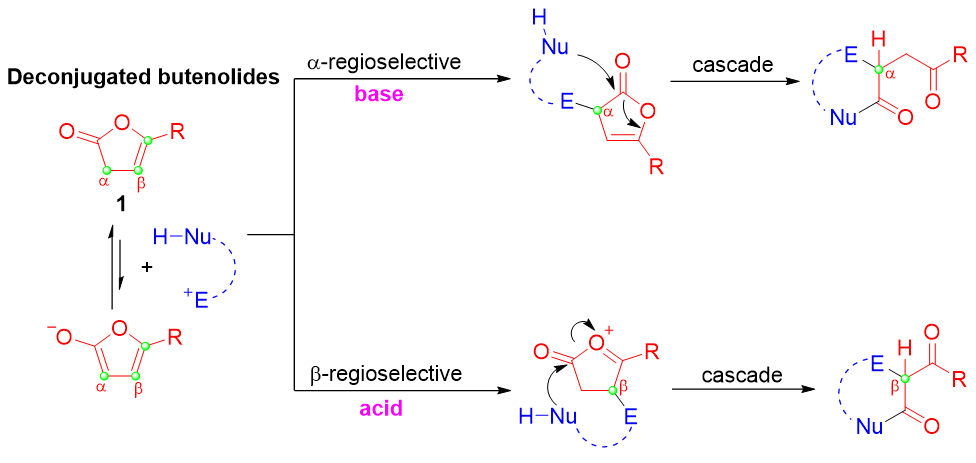
Key words: deconjugated butenolides; α-addition; β-addition; nucleophilic attack
| [1] | For selected reviews and examples, see: (a) Kitson, R.R.A.; Millemaggi, A.; Taylor, R., J. K. Angew. Chem., Int. Ed. 2009, 48, 9426. |
| [1] | (b) Lone, S. H.; Bhat, K. A.; Khuroo, M. A. Chem.-Biol. Interact. 2015, 240, 180. |
| [1] | (c) Yu, X.; Che, Z.; Xu, H. Chem.-Eur. J. 2017, 23, 4467. |
| [1] | (d) Curti, C.; Battistini, L.; Sartori, A.; Zanardi, F. Chem. Rev. 2020, 120, 2448. |
| [1] | (e) Choudhury, A. R.; Mukherjee, S. Chem. Soc. Rev. 2020, 49, 6755. |
| [1] | (f) Boeckman, R. K.; Pero, J. E.; Boehmler, D. J. J. Am. Chem. Soc. 2006, 128, 11032. |
| [1] | (g) Robichaud, J.; Tremblay, F. Org. Lett. 2006, 8, 597. |
| [1] | (h) Zhang, X.; Yin, Y.; Zhou, Y.; Zhu, T.; Wang, M.; Gao, H. Chin. J. Chem. 2022, 40, 617. |
| [1] | (i) You, J.-Q.; Liu, Y.-N.; Zhou, J.-S.; Sun, X.-Y.; Lei, C.; Mu, Q.; Li, J.-Y.; Hou, A.-J. Chin. J. Chem. 2022, 40, 2882. |
| [1] | (j) Zhang, H.; Nan, F. Chin. J. Chem. 2013, 31, 84. |
| [1] | (k) Tang, B.; Guan, A.; Zhao, Y.; Jiang, J.; Wang, M.; Zhou, L. Chin. J. Chem. 2017, 35, 1133. |
| [1] | (l) Wang, Y.; Chen, B.; He, X.; Gui, J. Chin. J. Chem. 2020, 38, 1339. |
| [2] | (a) Ma, S.-M.; Shi, Z.-J. Chin. J. Chem. 2001, 19, 1280. |
| [2] | (b) Wang, J. P.; Chang, X. H.; Tian, X. Z.; Chen, Q. H. Acta Chim. Sinica 2003, 61, 411 (in Chinese). |
| [2] | (王建平, 常新红, 田欣哲, 陈庆华, 化学学报, 2003, 61, 411.) |
| [2] | (c) Zhao, Y.; Jiang, S.; Guo, Y.-W.; Yao, Z.-J. Chin. J. Chem. 2005, 23, 173. |
| [2] | (d) Zhao, Y.; Tang, B.; Liu, X.; Li, W. Z.; Huang, M. Y.; Wang, M. A. Chin. J. Org. Chem. 2017, 37, 975 (in Chinese). |
| [2] | (赵宇, 汤博, 刘鑫磊, 李婉祯, 黄铭一, 王明安, 有机化学, 2017, 37, 975.) |
| [2] | (e) Li, X.; Zhu, K.; Han, Q.; Lu, X. X.; Li, M. J.; Ling, Y.; Duan, H. X. Chin. J. Org. Chem. 2022, 42, 202 (in Chinese). |
| [2] | (李想, 朱凯, 韩清, 路星星, 李明君, 凌云, 段红霞, 有机化学, 2022, 42, 202.) |
| [2] | (f) Zhang, Q.; Li, Y. H.; Xu, L. C.; Ma, H. Y.; Li, X. D.; Wang, M. A. Chin. J. Org. Chem. 2022, 42, 2438 (in Chinese). |
| [2] | (张倩, 李益豪, 许磊川, 马好运, 李向东, 王明安, 有机化学, 2022, 42, 2438.) |
| [3] | (a) Das, U.; Chen, Y.-R.; Tsai, Y.-L.; Lin, W. Chem.-Eur. J. 2013, 19, 7713. |
| [3] | (b) Rout, S.; Das, A.; Singh, V. K. Chem. Commun. 2017, 53, 5143. |
| [4] | (a) Adekenov, S. M.; Mukhametzhanov, M. N.; Kagarlitskii, A. D.; Kupriyanov, A. N. Khim. Prir. Soedin. 1982, 655. |
| [4] | (b) Zhangabylov, N. S.; Dederer, L. Y.; Gorbacheva, L. B.; Vasilêva, S. V.; Terekhov, A. S.; Adekenov, S. M. Pharm. Chem. J. 2004, 38, 651. |
| [5] | Aubert, S.; Katsina, T.; Arseniyadis, S. Org. Lett. 2019, 21, 2231. |
| [6] | For selected reviews, see: (a) Jusseau, X.; Chabaud, L.; Guillou, C. Tetrahedron 2014, 70, 2595. |
| [6] | (b) Roselló, M. S.; del Pozoa, C.; Fustero, S. Synthesis 2016, 48, 2553. |
| [6] | (c) Mao, B.; Fan?anas-Mastral, M.; Feringa, B. L. Chem. Rev. 2017, 117, 10502. |
| [6] | (d) Yin, Y.; Jiang, Z. ChemCatChem 2017, 9, 4306. |
| [6] | (e) Li, H.; Yin, L. Tetrahedron Lett. 2018, 59, 4121. |
| [7] | For selected examples on activated silyloxyfurans, see: (a) Redero, E.; Sandoval, C.; Bermejo, F. Tetrahedron 2001, 57, 9597. |
| [7] | (b) Singh, R. P.; Foxman, B. M.; Deng, L. J. Am. Chem. Soc. 2010, 132, 9558. |
| [7] | (c) Meshram, H. M.; Ramesh, P.; Reddy, B. C.; Sridhar, B.; Yadav, J. S. Tetrahedron 2011, 67, 3150. |
| [7] | (d) Mao, B.; Ji, Y.; Fananas-Mastral, M.; Caroli, G.; Meetsma, A.; Feringa, B. L. Angew. Chem., Int. Ed. 2012, 51, 3168. |
| [7] | (e) Chen, W.; Hartwig, J. F. J. Am. Chem. Soc. 2012, 134, 15249. |
| [7] | (f) Woyciechowska, M.; Forcher, G.; Buda, S.; Mlynarski, J. Chem. Commun. 2012, 48, 11029. |
| [7] | (g) Kong, S.; Fan, W.; Lyu, H.; Zhan, J.; Miao, X.; Miao, Z. Synth. Commun. 2014, 44, 936. |
| [7] | (h) Wang, Y.; Xing, F.; Xue, M.; Du, G.-F.; Guo, X.-H.; Huang, K.-W.; Dai, B. Synthesis 2016, 48, 79. |
| [7] | (i) Adamkiewicz, A.; W?glarz, I.; Butkiewicz, A.; Woyciechowska, M.; Mlynarski, J. Adv. Synth. Catal. 2020, 362, 667. |
| [8] | (a) Zhang, Y.; Yu, C.; Ji, Y.; Wang, W. Chem.-Asian J. 2010, 5, 1303. |
| [8] | (b) Huang, H.; Yu, F.; Jin, Z.; Li, W.; Wu, W.; Liang, X.; Ye, J. Chem. Commun. 2010, 46, 5957. |
| [8] | (c) Luo, J.; Wang, H.; Han, X.; Xu, L.-W.; Kwiatkowski, J.; Huang, K.-W.; Lu, Y. Angew. Chem., Int. Ed. 2011, 50, 1861. |
| [8] | (d) Yin, L.; Takada, H.; Lin, S.; Kumagai, N.; Shibasaki, M. Angew. Chem., Int. Ed. 2014, 53, 5327. |
| [9] | (a) Cui, H. L.; Huang, J. R.; Lei, J.; Wang, Z. F.; Chen, S.; Wu, L.; Chen, Y. C. Org. Lett. 2010, 12, 720. |
| [9] | (b) Wu, Y.; Singh, R. P.; Deng, L. J. Am. Chem. Soc. 2011, 133, 12458. |
| [9] | (c) Griswold, J. A.; Johnson, J. S. ACS Catal. 2019, 9, 11614. |
| [9] | (d) Yu, C.; Ji, P.; Zhang, Y.; Meng, X.; Wang, W. Org. Lett. 2021, 23, 7656. |
| [9] | (e) Dai, Z.-Y.; Wang, P.-S.; Gong, L.-Z. Chem. Commun. 2021, 57, 6748. |
| [10] | (a) Helberger, V. J. H.; Ulubey, S.; Civelekoglu, H. Justus Liebigs Ann. Chem. 1949, 561, 215. |
| [10] | (b) Karwa, S.; Gajiwala, V. M.; Heltzel, J.; Patil, S. K. R.; Lund, C. R. F. Catal. Today 2016, 263, 16. |
| [11] | Pelter, A.; Rowlands, M. Tetrahedron Lett. 1987, 28, 1203. |
| [12] | Marshall, J. A.; Wolf, M. A.; Wallace, E. M. J. Org. Chem. 1997, 62, 367. |
| [13] | (a) Osman, N. A.; Mahmoud, A. H.; Allara, M.; Niess, R.; Abouzid, K. A.; Marzo, V. D.; Abadi, A. H. Bioorg. Med. Chem. 2010, 18, 8463. |
| [13] | (b) Muratore, M. E.; Holloway, C. A.; Pilling, A. W.; Storer, R. I.; Trevitt, G.; Dixon, D. J. J. Am. Chem. Soc. 2009, 131, 10796. |
| [14] | Lima, C. G. S.; Monteiro, J. L.; Lima, T. Paix?o, M. W.; Corrêa, A. G. ChemSusChem. 2018, 11, 25. |
| [15] | For selected recent reports on the γ-addition of deconjugated butenolides, see: (a) Quintard, A.; Lefranc, A.; Alexakis, A. Org. Lett. 2011, 13, 1540. |
| [15] | (b) Zhou, L.; Lin, L.-L.; Ji, J.; Xie, M.-S.; Liu, X.-H.; Feng, X.-M. Org. Lett. 2011, 13, 3056. |
| [15] | (c) Zhang, W.; Tan, D.; Lee, R.; Tong, G.; Chen, W.; Qi, B.; Huang, K.-W.; Tan, C.-H.; Jiang, Z. Angew. Chem., Int. Ed. 2012, 51, 10069. |
| [15] | (d) Manna, M. S.; Kumar, V.; Mukherjee, S. Chem. Commun. 2012, 48, 5193. |
| [15] | (e) Manna, M. S.; Mukherjee, S. Chem.-Eur. J. 2012, 18, 15277. |
| [15] | (f) Ji, J.; Lin, L.-L.; Zhou, L.; Zhang, Y.-H.; Liu, Y.-B.; Liu, X.-H.; Feng X.-M. Adv. Synth. Catal. 2013, 355, 2764. |
| [15] | (g) Yang, D.; Wang, L.; Zhao, D.; Han, F.; Zhang, B.; Wang, R. Chem.-Eur. J. 2013, 19, 4691. |
| [16] | For selected reports on the γ-addition of deconjugated butenolides, see: (a) Guo, Y.-L.; Jia, L.-N.; Peng, L.; Qi, L.-W.; Zhou, J.; Tian, F.; Xu, X.-Y.; Wang, L. -X. RSC Adv. 2013, 3, 16973. |
| [16] | (b) Kumar, V.; Ray, B.; Rathi, P.; Mukherjee, S. Synthesis 2013, 45, 1641. |
| [16] | (c) Manna, M. S.; Mukherjee, S. Chem. Sci. 2014, 5, 1627. |
| [16] | (d) Li, X.; Lu, M.; Dong, Y.; Wu, W.; Qian, Q.; Ye, J.; Dixon, D. J. Nat. Commun. 2014, 4, 4479. |
| [16] | (e) Wang, Z.-H.; Wu, Z.-J.; Huang, X.-Q.; Yue, D.-F.; You, Y.; Xu, X.-Y.; Zhang, X.-M.; Yuan, W.-C. Chem. Commun. 2015, 51, 15835. |
| [16] | (f) Guo, H.; Xing, F.; Du, G.-F.; Huang, K.-W.; Dai, B.; He, L. J. Org. Chem. 2015, 80, 12606. |
| [17] | For selected reports on the γ-addition of deconjugated butenolides, see: (a) Sekikawa, T.; Kitaguchi, T.; Kitaura, H.; Minami, T.; Hatanaka, Y. Org. Lett. 2015, 17, 3026. |
| [17] | (b) Lagoutte, R.; Besnard, C.; Alexakis, A. Eur. J. Org. Chem. 2016, 4372. |
| [17] | (c) Simlandy, A. K.; Mukherjee, S. Org. Biomol. Chem. 2016, 14, 5659. |
| [17] | (d) Tang, Q.; Lin, L.-L.; Ji, J.; Hu, H.-P.; Liu, X.-H.; Feng, X.-M. Chem.-Eur. J. 2017, 23, 16447. |
| [17] | (e) Ji, J.; Lin, L.-L.; Tang, Q.; Kang, T.-F.; Liu, X.-H.; Feng, X.-M. ACS Catal. 2017, 7, 3763. |
| [17] | (f) Trost, B. M.; Gnanamani, E.; Tracy, J. S.; Kalnmals, C. J. Am. Chem. Soc. 2017, 139, 18198. |
| [17] | (g) Rout, S.; Joshi, H.; Singh, V. K. Org. Lett. 2018, 20, 2199. |
| [18] | For selected reports on the γ-addition of deconjugated butenolides, see: (a) Sakai, T.; Hirashima, S.; Matsushima, Y.; Nakano, T.; Ishii, D.; Yamashita, Y.; Nakashima, K.; Koseki, Y.; Miura, T. Org. Lett. 2019, 21, 2606. |
| [19] | Jefford, C. W.; Jaggi, D.; Boukouvalas, J. J. Chem. Soc., Chem. Commun. 1988, 1595. |
| [20] | Egorova, A. Y.; Timofeeva, Z. Y. Russ. J. Gen. Chem. 2003, 73, 655. |
| [21] | Griswold, J. A.; Horwitz, M. A.; Leiva, L. V.; Johnson, J. S. J. Org. Chem. 2017, 82, 2276. |
| [22] | (a) Mengel, A.; Reiser, O. Chem. Rev. 1999, 99, 1191. |
| [22] | (b) Evans, D. A.; Siska, S. J.; Cee, V. J. Angew. Chem., Int. Ed. 2003, 42, 1761. |
| [22] | (c) Cee, V. J.; Cramer, C. J.; Evans, D. A. J. Am. Chem. Soc. 2006, 128, 2920. |
| [23] | Yang, M.; Chen, C.; Yi, X.; Li, Y.; Wu, X.; Li, Q.; Ban, S. Org. Biomol. Chem. 2019, 17, 2883. |
| [24] | Li, Feng.; Wang, J.; Pei, W.; Ma, H.; Li, H.; Cui, M.; Peng, S.; Wang, S.; Liu, L. Tetrahedron Lett. 2018, 59, 3010. |
| [25] | Trost, B. M.; Joey Hung, C.-I.; Scharf, M. J. Angew. Chem., Int. Ed. 2018, 57, 11408. |
| [26] | Huang, Z-Y.; Yang, H.; Zhou, L.; Li, Q.-H.; Zhao, Z.-G. Tetrahedron. 2022, 112, 132740. |
| [27] | Wu, Bo.; Yu, Z.; Gao, X.; Lan, Y.; Zhou, Y.-G. Angew. Chem., Int. Ed. 2017, 56, 4006. |
| [28] | (a) Green, A. G.; Liu, P.; Merlic, C. A.; Houk, K. N. J. Am. Chem. Soc. 2014, 136, 4575. |
| [28] | (b) Wang, T.; Yu, Z.; Hoon, D. L.; Phee, C. Y.; Lan, Y.; Lu, Y. J. Am. Chem. Soc. 2016, 138, 265. |
| [29] | Wang, Y.-N.; Xiong, Q.; Lu, L.-Q.; Zhang, Q.-L.; Wang, Y.; Lan, Y.; Xiao, W.-J. Angew. Chem., Int. Ed. 2019, 58, 11013. |
| [30] | Xu, Y.-W.; Hu, X.-P. Org. Lett. 2019, 21, 8091. |
| [31] | Hattori, G.; Sakata, K.; Matsuzawa, H.; Tanabe, Y.; Miyake, Y.; Nishibayashi, Y. J. Am. Chem. Soc. 2010, 132, 10592. |
| [32] | Zhao, J.-Q.; Luo, S.-W.; Zhang, X.-M.; Xu, X.-Y.; Zhou, M.-Q.; Yuan, W.-C. Tetrahedron 2017, 73, 5444. |
| [33] | Sharma, B. M.; Shinde, D. R.; Jain, R.; Begari, E.; Satbhaiya, S.; Gonnade, R. G.; Kumar, P. Org. Lett. 2018, 20, 2787. |
| [34] | Masuyama, Y.; Kobayashi, Y.; Yanagi, R.; Kurusu, Y. Chem. Lett. 1992, 2039. |
| [35] | Isambert, N.; Cruz, M.; Arévalo, M. J.; Gómez, E.; Lavilla, R. Org. Lett. 2007, 9, 4199. |
| [36] | Huo, C.; Yuan, Yong. J. Org. Chem. 2015, 80, 12704. |
| [37] | Huo, C.; Yuan, Y.; Chen, F.; Tang, J.; Wang, Y. Org. Lett. 2015, 17, 4208. |
| [38] | Zhou, H.; Yang, X.; Li, S.; Zhu, Y.; Li, Y.; Zhang, Y. Org. Biomol. Chem. 2018, 16, 6728. |
| [39] | (a) For reviews on nitrosocarbonyl chemistry, see: Palmer, L. I.; Frazier, C. P.; Read De Alaniz, J. Synthesis 2014, 46, 269. |
| [39] | (b) Maji, B.; Yamamoto, H. Bull. Chem. Soc. Jpn. 2015, 88, 753. |
| [39] | (c) Memeo, M. G.; Quadrelli, P. Chem. Rev. 2017, 117, 2108. |
| [39] | (d) Dana, S.; Ramakrishna, I.; Baidya, M. Synthesis 2017, 49, 3281. |
| [40] | Mallik, S.; Bhajammanavar, V.; Baidya, M. Org. Lett. 2020, 22, 1437. |
| [41] | (a) Payette, J. N.; Yamamoto, H. J. Am. Chem. Soc. 2008, 130, 12276. |
| [41] | (b) Maji, B.; Yamamoto, H. Angew. Chem., Int. Ed. 2014, 53, 14472. |
/
| 〈 |
|
〉 |