

羰基α-位胺化反应研究进展
收稿日期: 2023-01-06
修回日期: 2023-02-19
网络出版日期: 2023-04-10
基金资助
黑龙江省自然科学基金(LH2022B003); 中央高校基本科研业务费专项资金(41421029); 中央高校基本科研业务费专项资金(2572020BU03)
Research Progress of Carbonyl α-Position Amination
Received date: 2023-01-06
Revised date: 2023-02-19
Online published: 2023-04-10
Supported by
The Natural Science Foundation of Heilongjiang Province(LH2022B003); The Fundamental Research Funds for the Central Universities(41421029); The Fundamental Research Funds for the Central Universities(2572020BU03)
吴文倩 , 陈春霞 , 彭进松 , 李占宇 . 羰基α-位胺化反应研究进展[J]. 有机化学, 2023 , 43(8) : 2743 -2763 . DOI: 10.6023/cjoc202301005
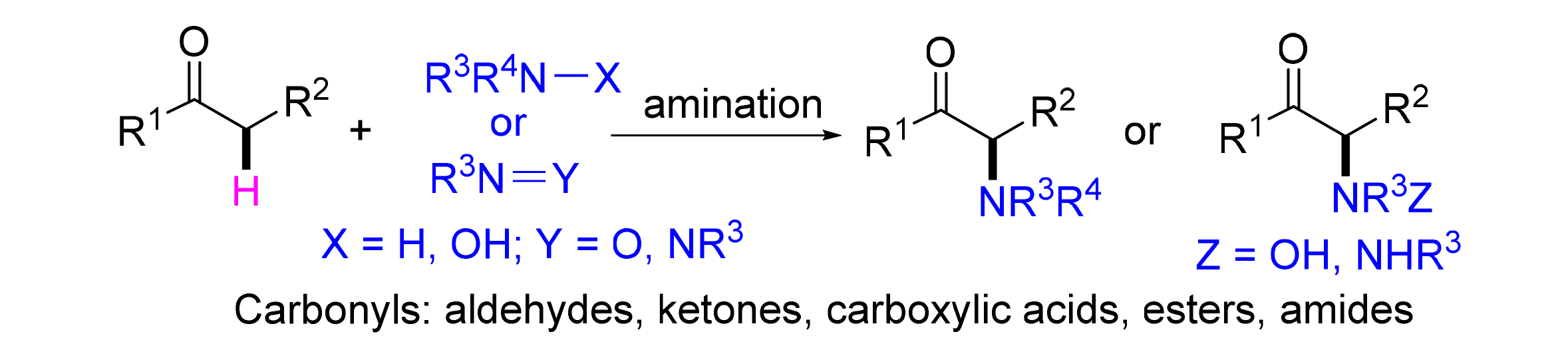
α-Amino carbonyl derivatives are an important class of molecular backbones found in many pharmaceuticals and natural product molecules, meanwhile, they are also an essential kind of intermediates for the synthesis of many organic molecules. To develop simple and efficient method for the synthesis of structurally diversity α-amino carbonyl derivatives of great importance, the amination reactions of carbonyl α-position hydrocarbon bond in recent decades are classified and summarized based on the different activation patterns experienced by the reactions. These reactions are mainly divided into four categories: electrophilic amination, oxidative amination, halide mediated amination, and electrochemical amination.
| [1] | Meltzer, P. C.; Butler, D.; Deschamps, J. R.; Madras, B. K. J. Med. Chem. 2006, 49, 1420. |
| [2] | Carroll, F. I.; Blough, B. E.; Abraham, P.; Mills, A. C.; Holleman, J. A.; Wolckenhauer, S. A.; Decker, A. M.; Landavazo, A.; McElroy, K. T.; Navarro, H. A.; Gatch, M. B.; Forster, M. J. J. Med. Chem. 2009, 52, 6768. |
| [3] | Silverstone, T. Drugs 1992, 43, 820. |
| [4] | Marquis, R. W.; Ru, Y.; Yamashita, D. S.; Oh, H. J.; Yen, J.; Thompson, S. K.; Carr, T. J.; Levy, M. A.; Tomaszek, T. A.; Ijames, C. F.; Smith, W. W.; Zhao, B.; Janson, C. A.; Abdel-Meguid, S. S.; DAlessio, K. J.; McQueney, M. S.; Veber, D. F. J. Bioorg. Med. Chem. 1999, 7, 581. |
| [5] | Nchinda, A. T.; Chibale, K.; Redelinghuys, P.; Sturrock, E. D. Bioorg. Med. Chem. Lett. 2006, 16, 4612. |
| [6] | Knorr, L. J. Eur. J. Org. Chem. 1888, 1888, 357. |
| [7] | Sorrell, T. N.; Allen, W. E. J. Org. Chem. 1994, 59, 1589. |
| [8] | Chiba, T.; Sakagami, H.; Murata, M.; Okimoto, M. J. Org. Chem. 1995, 60, 6764. |
| [9] | Nadkarni, D.; Hallissey, J.; Mojica, C. J. Org. Chem. 2003, 68, 594. |
| [10] | Williams, R. M.; Hendrix, J. A. J. Chem. Rev. 1992, 92, 889. |
| [11] | Xu, J.; Green, A. P.; Turner, N. J. Angew. Chem., Int. Ed. 2018, 57, 16760. |
| [12] | Fisher, L. E.; Muchowski, J. M. Org. Prep. Proced. Int. 1990, 22, 399. |
| [13] | Allen, L. A. T.; Raclea, R. C.; Natho, P.; Parsons, P. J. Org. Biomol. Chem. 2021, 19, 498. |
| [14] | Li, J. Z.; Zhang, W. K.; Ge, G. P.; Zheng, H. X.; Wei, W. T. Org. Biomol. Chem. 2021, 19, 7333. |
| [15] | Greck, C.; Drouillat, B.; Thomassigny, C. Eur. J. Org. Chem. 2004, 2004, 1377. |
| [16] | Erdik, E. Tetrahedron. 2004, 60, 8747. |
| [17] | Greck, C.; Bischoff, l.; Ferreira, F.; Pinel, C.; Piveteau, E.; Genet, J. P. J. Synlett. 1993, 475. |
| [18] | Bulman Page, P. C.; McKenzie, M. J.; Allina, S. M.; Buckle, D. R. J. Tetrahedron 2000, 56, 9683. |
| [19] | Asano, T.; Moritani, M.; Nakajima, M.; Kotani, S. Tetrahedron 2017, 73, 5975. |
| [20] | Evans, D. A.; Nelson, S. G. J. Am. Chem. Soc. 1997, 119, 6452. |
| [21] | Juhl, K.; J?rgensen, K. A. J. Am. Chem. Soc. 2002, 124, 2420. |
| [22] | Marigo, M.; Juhl, K.; J?rgensen, K. A. J. Angew. Chem. 2003, 115, 12. |
| [23] | Ma, S.; Jiao, N.; Zheng, Z.; Ma, Z.; Lu, Z. Org. Lett. 2004, 6, 2193. |
| [24] | Xiao, X.; Lin, L.; Lian, X.; Liu, X.; Feng, X. Org. Chem. Front. 2016, 3, 809. |
| [25] | Comelles, J.; Pericas, A.; Moreno-Manas, M.; Vallribera, A.; Drudis-Sole, G.; Lledos, A.; Parella, T.; Roglans, A.; Garcia-Granda, S.; Roces-Fernandez, L. J. Org. Chem. 2007, 72, 2077. |
| [26] | Pericas, A.; Jimenez, R.; Granados, A.; Shafir, A.; Vallribera, A.; Roglans, A.; Molins, E. ChemistrySelect 2016, 1, 4305. |
| [27] | Naganawa, Y.; Komatsu, H.; Nishiyama, H. Chem. Lett. 2015, 44, 1652. |
| [28] | B?gevig, A.; Juhl, K.; Kumaragurubaran, N.; Zhuang, W.; J?rgensen, K. A. J. Angew. Chem., Int. Ed. 2002, 41, 10. |
| [29] | Kumaragurubaran, N.; Juhl, K.; Zhuang, W.; B?gevig, A.; J?rgensen, K. A. J. J. Am. Chem. Soc. 2002, 124, 6254. |
| [30] | Iwamura, H.; Wells, D. H.; Mathew, S. P.; Klussmann, M.; Armstrong, A.; Blackmond, D. G. J. J. Am. Chem. Soc. 2004, 126, 16312. |
| [31] | Franzen, J.; Marigo, M.; Fielenbach, D.; Wabnitz, T. C.; Kj?r- sgaard, A.; J?rgensen, K. A. J. J. Am. Chem. Soc. 2005, 127, 18296. |
| [32] | Suri, J. T.; Steiner, D. D.; Barbas, C. F. Org. Lett. 2005, 7, 3885. |
| [33] | Desmarchelier, A.; Yalgin, H.; Coeffard, V.; Moreau, X.; Greck, C. Tetrahedron Lett. 2011, 52, 4430. |
| [34] | Liu, C.; Zhu, Q.; Huang, K.; Lu, Y. Org. Lett. 2011, 13, 2638. |
| [35] | Zhou, F.; Zeng, X.; Wang, C.; Zhao, X.; Zhou, J. Chem. Commun. 2013, 49, 2022. |
| [36] | Lim, Y. J.; Kim, D. Y. B. Bull. Korean. Chem. Soc. 2013, 34, 1955. |
| [37] | Xu, C.; Zhang, L.; Luo, S. J. Org. Chem. 2014, 79, 11517. |
| [38] | List, B.; Shevchenko, G.; Pupo, G. Synlett 2015, 26, 1413. |
| [39] | Odagi, M.; Yamamoto, Y.; Nagasawa, K. Beilstein J. Org. Chem. 2016, 12, 198. |
| [40] | Shang, M.; Wang, X.; Koo, S. M.; Youn, J.; Chan, J. Z.; Yao, W.; Hastings, B. T.; Wasa, M. J. Am. Chem. Soc. 2017, 139, 95. |
| [41] | Morisawa, T.; Sawamura, M.; Shimizu, Y. Org. Lett. 2019, 21, 7466. |
| [42] | Morrill, L. C.; Lebl, T.; Slawina, A. M. Z.; Smith, A. D. Chem. Sci. 2012, 3, 2088. |
| [43] | Zhang, T.; Cheng, L.; Liu, L.; Wang, D.; Chen, Y. J. Tetrahedron: Asymmetry 2010, 21, 2800. |
| [44] | Companyo, X.; Valero, G.; Pineda, O.; Calvet, T.; Font-Bardia, M.; Moyano, A.; Rios, R. Org. Biomol. Chem. 2012, 10, 431. |
| [45] | Jia, L. N.; Huang, J.; Peng, L.; Wang, L. L.; Bai, J. F.; Tian, F.; He, G. Y.; Xu, X. Y.; Wang, L. X. Org. Biomol. Chem. 2012, 10, 236. |
| [46] | Ohmatsu, K.; Ando, Y.; Nakashima, T.; Ooi, T. Chem 2016, 1, 802. |
| [47] | Shen, K.; Liu, X.; Wang, G.; Lin, L.; Feng, X. Angew. Chem., Int. Ed. 2011, 50, 4684. |
| [48] | Sandoval, D.; Frazier, C. P.; Bugarin, A.; Read de Alaniz, J. J. Am. Chem. Soc. 2012, 134, 18948. |
| [49] | Murru, S.; Lott, C. S.; Fronczek, F. R.; Srivastava, R. S. Org. Lett. 2015, 17, 2122. |
| [50] | Moriarty, R. M.; Vaid, R. K.; Ravikumar, V. T.; Vaid, B. K.; Hopkins, T. E. J. Tetrahedron 1988, 6, 1603. |
| [51] | Lee, J. C.; Kim, S.; Shin, W. C. Synth. Commun. 2000, 30, 4271. |
| [52] | Sun, Y.; Fan, R. Chem. Commun. 2010, 46, 6834. |
| [53] | Kamble, D. A.; Karabal, P. U.; Chouthaiwale, P. V.; Sudalai, A. Tetrahedron Lett. 2012, 53, 4195. |
| [54] | Gao, W. C.; Jiang, S.; Wang, R. L.; Zhang, C. Chem. Commun. 2013, 49, 4890. |
| [55] | Jiang, Q.; Xu, B.; Zhao, A.; Jia, J.; Liu, T.; Guo, C. J. Org. Chem. 2014, 79, 8750. |
| [56] | Lv, Y.; Li, Y.; Xiong, T.; Lu, Y.; Liu, Q.; Zhang, Q. Chem. Commun. 2014, 50, 2367. |
| [57] | Wang, D.; Lu, X.; Sun, S.; Yu, H.; Su, H.; Wu, Y.; Zhong, F. Eur. J. Org. Chem. 2019, 2019, 6028. |
| [58] | Zhong, F.; Chen, G.; Lu, Y. Org. Lett. 2022, 24, 842. |
| [59] | Takeda, M.; Maejima, S.; Yamaguchi, E.; Itoh, A. Tetrahedron Lett. 2021, 77, 153251. |
| [60] | Li, K.; Li, Q.; Shi, Q.; He, Y.; Yu, W.; Chang, J. Asian J. Org. Chem. 2022, 11, e202200268. |
| [61] | Yakura, T.; Yoshimoto, Y.; Ishida, C. J. Chem. Pharm. Bull. 2007, 55, 1385. |
| [62] | Ton, T. M.; Himawan, F.; Chang, J. W.; Chan, P. W. Chemistry 2012, 18, 12020. |
| [63] | Tokumasu, K.; Yazaki, R.; Ohshima, T. J. Am. Chem. Soc. 2016, 138, 2664. |
| [64] | Evans, R. W.; Zbieg, J. R.; Zhu, S.; Li, W.; MacMillan, D. W. J. Am. Chem. Soc. 2013, 135, 16074. |
| [65] | McDonald, S. L.; Wang, Q. Chem. Commun. 2014, 50, 2535. |
| [66] | Jia, W. G.; Li, D. D.; Dai, Y. C.; Zhang, H.; Yan, L. Q.; Sheng, E. H.; Wei, Y.; Mu, X. L.; Huang, K. W. Org. Biomol. Chem. 2014, 12, 5509. |
| [67] | Tran, T. V.; Le, H. T. N.; Ha, H. Q.; Duong, X. N. T.; Nguyen, L. H. T.; Doan, T. L. H.; Nguyen, H. L.; Truong, T. Catal. Sci. Technol. 2017, 7, 3453. |
| [68] | Rossi, L. I.; Krapacher, C. R.; Granados, A. M. Mol. Catal. 2020, 493, 111058. |
| [69] | Yu, J.; Liu, S. S.; Cui, J.; Hou, X. S.; Zhang, C. J. Org. Lett. 2012, 3, 832. |
| [70] | Deng, Q. H.; Bleith, T.; Wadepohl, H.; Gade, L. H. J. Am. Chem. Soc. 2013, 135, 5356. |
| [71] | Mudaliar, S. S.; Patel, A. P.; Patel, J. J.; Chikhalia, K. H. Tetrahedron Lett. 2018, 59, 734. |
| [72] | Lu, H.; Lang, K.; Jiang, H.; Wojtas, L.; Zhang, X. P. Chem. Sci. 2016, 7, 6934. |
| [73] | (a) Kaiser, D.; Maulide, N. J. Org. Chem. 2016, 81, 4421. |
| [73] | (b) Tona, V.; de la Torre, A.; Padmanaban, M.; Ruider, S.; Gonzalez, L.; Maulide, N. J. Am. Chem. Soc. 2016, 138, 8348. |
| [74] | Rezayee, N. M.; Rusbjerg, M.; Marx, M.; Linde, S. T.; J?rgensen, K. A. J. Org. Chem. 2022, 87, 1756. |
| [75] | Wei, Y.; Lin, S.; Liang, F. Org. Lett. 2012, 14, 4202. |
| [76] | Kumar, Y.; Jaiswal, Y.; Thakur, R.; Kumar, A. ChemistrySelect 2018, 3, 5614. |
| [77] | Lamani, M.; Prabhu, K. R. Chemistry 2012, 18, 14638. |
| [78] | Liang, S.; Zeng, C. C.; Tian, H. Y.; Sun, B. G.; Luo, X. G.; Ren, F. Z. J. Org. Chem. 2016, 81, 11565. |
/
| 〈 |
|
〉 |