

可见光诱导的自由基环化反应构建4-芳基-1,2-二氢萘类化合物
收稿日期: 2023-02-05
修回日期: 2023-04-20
网络出版日期: 2023-05-06
基金资助
广西科技大学人才启动基金(03190285); 广西科技基地与人才专项基金(桂科AD20238008)
Visible Light-Induced Radical Cyclization for the Construction of 4-Aryl-1,2-dihydronaphthalenes
Received date: 2023-02-05
Revised date: 2023-04-20
Online published: 2023-05-06
Supported by
Start-Up Funding from Guangxi University of Science and Technology(03190285); Guangxi Science and Technology Base and Talent Special Project Fund (AD20238008)
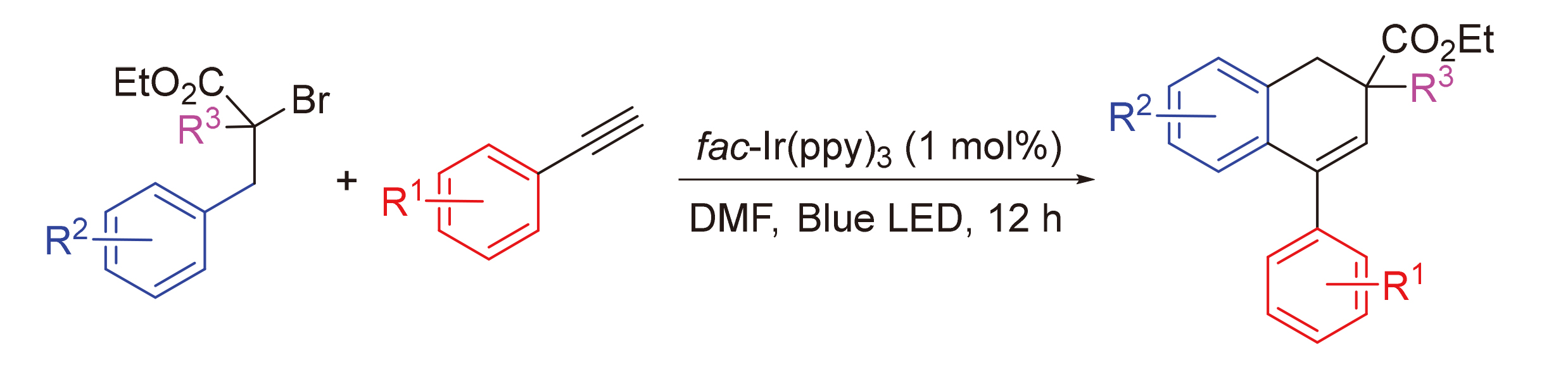
报道了一种高效的可见光诱导的自由基环化合成4-芳基-1,2-二氢萘类化合物的方法. 与传统的亲核加成反应相比, 该方法条件温和, 在室温条件下, 用7 W蓝光照射, 以fac-Ir(ppy)3为光催化剂, 利用简单易得的溴代丙二酸酯类化合物做自由基前体, 与不同类型的单取代芳基乙炔进行自由基串联环化反应, 得到目标产物. 该方法后处理简便. 底物适用性较广, 对不同的溴代丙二酸酯和不同的炔烃都有很好的适用性.
关键词: 可见光诱导; 4-芳基-1,2-二氢萘; 自由基串联环化
樊思捷 , 董武恒 , 梁彩云 , 王贵超 , 袁瑶 , 尹作栋 , 张兆国 . 可见光诱导的自由基环化反应构建4-芳基-1,2-二氢萘类化合物[J]. 有机化学, 2023 , 43(9) : 3277 -3286 . DOI: 10.6023/cjoc202302005
An efficient visible light-induced radical tandem cyclization method for the synthesis of 4-aryl-1,2-dihydronaph- thalenes is reported. The target products were obtained by the radical tandem cyclization of different types of monosubstituted aryl acetylenes with simple bromomalonates as radical precursors. Compared to conventional nucleophilic addition reactions, the method was obtained under mild conditions by irradiation with 7 W blue light at room temperature using fac-Ir(ppy)3 as a photocatalyst. The reaction conditions are mild and the post-treatment is simple. This free radical triggered tandem cyclisation avails readily available bromomalonate esters and aryl alkynes as the starting materials. The method is very suitable for a wide range of substrates with good applicability to different bromomalonates and different alkynes.

| [1] | Walkinshaw A. J.; Xu W.; Suero M. G.; Gaunt M. J. J. Am. Chem. Soc. 2013, 135, 12532. |
| [2] | Bering L.; Jeyakumar K.; Antonchick A. P. Org. Lett. 2018, 20, 3911. |
| [3] | Navaratne P. V.; Grenning A. J. Org. Biomol. Chem. 2017, 15, 69. |
| [4] | Xiao J.; Cong X. W.; Yang G. Z.; Wang Y. W.; Peng Y. Org. Lett. 2018, 20, 1651. |
| [5] | Charlton J. L.; Oleschuk C. J.; Chee G.-L. J. Org. Chem. 1996, 61, 3452. |
| [6] | Tian G.; Fedoseev P.; Van der Eycken E. V. Chem.-Eur. J. 2017, 23, 5224. |
| [7] | Lei C.; Yip Y. J.; Zhou J. S. J. Am. Chem. Soc. 2017, 139, 6086. |
| [8] | Stavber G.; Zupan M.; Stavber S. Tetrahedron Lett. 2006, 47, 8463. |
| [9] | Yue G. L.; Wei J. Y.; Qiu D.; Mo F.-Y. Acta Chim. Sinica 2022, 80, 956. (in Chinese) |
| [9] | (岳广禄, 魏婧瑶, 邱頔, 莫凡洋, 化学学报, 2022, 80, 956.) |
| [10] | Martínez A. G.; Barcina J. O.; Colorado Heras M. D. R.; Fresno De Fresno Cerezo á. Organometallics 2001, 20, 1020. |
| [11] | Campagne J.-M.; Dal Zotto C.; Wehbe J.; Virieux D. Synlett 2008, 2033. |
| [12] | Cheng L.; Zhou Q. L. Acta Chim. Sinica 2020, 78, 1017. (in Chinese) |
| [12] | (程磊, 周其林, 化学学报, 2020, 78, 1017.) |
| [13] | Qiu Z.; Xie Z. Angew. Chem., Int. Ed. 2009, 48, 5729. |
| [14] | Sun C. L.; Wang Y.; Zhou X.; Wu Z. H.; Li B. J.; Guan B. T.; Shi Z. J. Chem.-Eur. J. 2010, 20, 5844. |
| [15] | Novikov R. A.; Borisov D. D.; Tarasova A. V.; Tkachev Y. V.; Tomilov Y. V. Angew. Chem., Int. Ed. 2018, 57, 10293. |
| [16] | (a) Prier C. K.; Rankic D. A.; MacMillan D. W. C. Chem. Rev. 2013, 113, 5322. |
| [16] | (b) Fagnoni D. M.; Albini A. Chem. Soc. Rev. 2013, 42, 97. |
| [17] | (a) Fukuzumi S.; Ohkubo K. Org. Biomol. Chem. 2014, 12, 6059. |
| [17] | (b) Furst L.; Narayanam J. M.; Stephenson C. R. Angew. Chem., Int. Ed. 2011, 50, 9655. |
| [17] | (c) Gu X.; Lu P.; Fan W.; Li P.; Yao Y. Org. Biomol. Chem. 2013, 11, 7088. |
| [17] | (d) Hari D. P.; Schroll P.; K?nig B. J. Am. Chem. Soc. 2012, 134, 2958. |
| [17] | (e) Kalyani D.; McMurtrey K. B.; Neufeldt S. R.; Sanford M. S. J. Am. Chem. Soc. 2011, 133, 18566. |
| [18] | Deng G. B.; Wang Z. Q.; Xia J. D.; Qian P. C.; Song R. J.; Hu M.; Gong L. B.; Li J. H. Angew. Chem., Int. Ed. 2013, 52, 1535. |
| [19] | Liu J.; Wei Y.; Shi M. Org. Chem. Front. 2021, 8, 94. |
| [20] | Dong W.; Yuan Y.; Gao X.; Keranmu M.; Li W.; Xie X.; Zhang Z. Org. Lett. 2018, 20, 5762. |
| [21] | Dong W.; Yuan Y.; Xie X.; Zhang Z. Org. Lett. 2020, 22, 528. |
| [22] | Dong W.; Yuan Y.; Liang C.; Wu F.; Zhang S.; Xie X.; Zhang Z. J. Org. Chem. 2021, 86, 3697. |
| [23] | Citterio A.; Sebastiano R.; Maronati A.; Santi R.; Bergamini F. J. Chem. Soc., Chem. Commun. 1994, 1517. |
| [24] | Yuan Y.; Zhang S. Y.; Dong W. H.; Wu F.; Xie X. M.; Zhang Z. G. Adv. Synth. Catal. 2021, 363, 4216. |
| [25] | (a) Huo H.; Shen X.; Wang C.; Zhang L.; R?se P.; Chen L. A.; Harms K.; Marsch M.; Hilt G.; Meggers E. Nature 2014, 515, 100. |
| [25] | (b) Larraufie M. H.; Pellet R.; Fensterbank L.; Goddard J. P.; Lacote E.; Malacria M.; Ollivier C. Angew. Chem., Int. Ed. 2011, 50, 4463. |
| [25] | (c) Neumann M.; Fuldner S.; Konig B.; Zeitler K. Angew. Chem., Int. Ed. 2011, 50, 951. |
| [25] | (d) Nguyen J. D.; D'Amato E. M.; Narayanam J. M. R.; Stephenson C. R. J. Nat. Chem. 2012, 4, 854. |
| [26] | (a) Nguyen J. D.; Tucker J. W.; Konieczynska M. D.; Stephenson C. R. J. J. Am. Chem. Soc. 2011, 133, 4160. |
| [26] | (b) Nicewicz D. A.; MacMillan D. W. C. Science 2008, 322, 77. |
/
| 〈 |
|
〉 |