

PPh3-促进邻炔基硝基苯合成3-羟基-2-吲哚酮
收稿日期: 2023-04-13
修回日期: 2023-06-05
网络出版日期: 2023-06-14
基金资助
江苏省自然科学基金(BK20171449)
PPh3-Mediated Synthesis of 3-Hydroxy-2-oxindoles from o-Alkynylnitrobenzenes
Received date: 2023-04-13
Revised date: 2023-06-05
Online published: 2023-06-14
Supported by
Natural Science Foundation of Jiangsu Province-Grants(BK20171449)
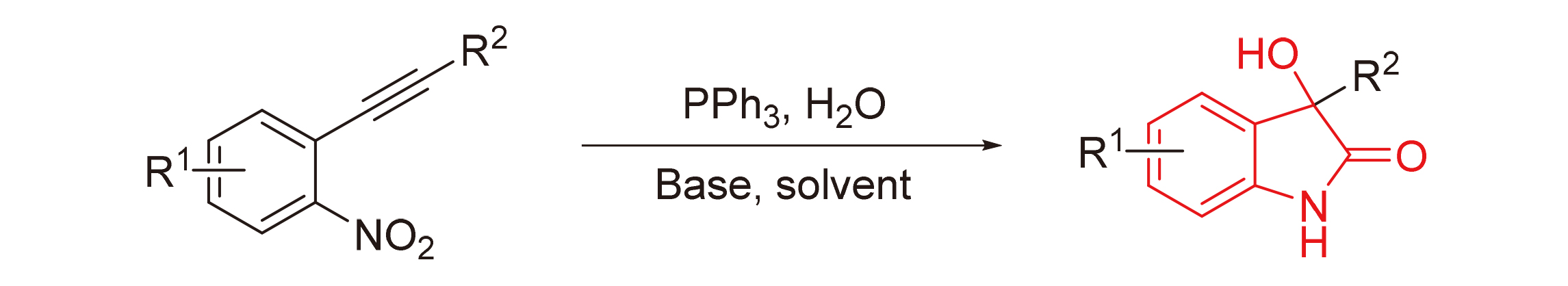
3-羟基-2-吲哚酮核心结构在合成化学和药物化学中都具有重要的意义. 发现了一种新的、有效的由邻炔基硝基芳烃制备3-羟基-2-吲哚酮的碱促进方法, 该反应可在不含过渡金属的条件下进行. 对各种邻炔基硝基芳烃进行测定, 并以中等至良好的产率获得所需的产物. 由三苯基膦引发的Wittig反应和随后的偶姻重排构成了主要的反应过程.
关键词: PPh3; Wittig反应; 3-羟基-2-吲哚酮; 邻炔基硝基
赵雪纯 , 樊辉 , 徐瑶 , 廖小铭 , 张小祥 . PPh3-促进邻炔基硝基苯合成3-羟基-2-吲哚酮[J]. 有机化学, 2023 , 43(11) : 3997 -4002 . DOI: 10.6023/cjoc202304017
The 3-hydroxy-2-oxindole core structure is of considerable significance in both synthetic and medicinal chemistry. A novel and efficient base-promoted method for the preparation of 3-hydroxy-2-oxindoles from o-alkynylnitroarenes has been discovered. The reaction could be conducted under transition metal free conditions. Various o-alkynylnitroarenes were checked and the desired products were obtained in moderate to good yields. The Wittig-like reaction triggered by the PPh3 and the subsequent acyloin rearrangement constituted the main reaction process.

Key words: PPh3; Wittig reaction; 3-hydroxy-2-oxindoles; o-alkynylnitroarenes
| [1] | (a) Lin, M. J.; Zhu, L.; Xia, J. J.; Yu, Y. H.; Chen, J. X.; Mao, Z. F.; Huang, X. L. Adv. Synth. Catal. 2018, 360, 2280. |
| [1] | (b) Yu, Y. H.; Zhu, L.; Liao, Y.; Mao, Z. F.; Huang, X. L. Adv. Synth. Catal. 2016, 358, 1059. |
| [1] | (c) Kenny, R. T.; Liu, F. Asian J. Org. Chem. 2022, 11. |
| [1] | (d) Ma, L. Y.; Yu, Y. H.; Xin, L. T.; Zhu, L.; Xia, J. J.; Ou, P. C.; Huang, X. L. Adv. Synth. Catal. 2021, 363, 2573. |
| [1] | (e) Day, D. P.; Chan, P. W. H. Adv. Synth. Catal. 2016, 358, 1368. |
| [1] | (f) Jin, J. W.; Zhao, Y. C.; Sze, E. M. L.; Kothandaraman, P.; Chan, P. W. H. Adv. Synth. Catal. 2018, 360, 4744. |
| [1] | (g) Zhao, Y. C.; Henry, S. A.; Jin, J. W.; Clarkson, G. J.; Chan, P. W. H. Asian J. Org. Chem. 2019, 8, 1029. |
| [1] | (h) Zheng, Y.; Qian, S. C.; Xu, P. C.; Ma, T. T.; Huang, S. L. Adv. Synth. Catal. 2022, 364, 3800. |
| [1] | (i) Meng, X. T.; Xu, H. H.; Liu, R.; Zheng, Y.; Huang, S. L. Green Chem. 2022, 24, 4754. |
| [1] | (j) Huang, Y.; Chen, D. F.; Zheng, Y.; Huang, S. L. Chemistryselect, 2022, 7. |
| [1] | (k) Song, L. N.; Tian, X. H.; Han, C. Y.; Amanpur, M.; Rominger, F.; Hashmi, A. S. K. Org. Chem. Front. 2021, 8, 3314. |
| [1] | (l) Greiner, L. C.; Inuki, S.; Arichi, N.; Oishi, S.; Suzuki, R.; Iwai, T.; Sawamura, M.; Hashmi, A. S. K.; Ohno, H. Chem.-Eur. J. 2021, 27, 12992. |
| [1] | (m) Lustosa, D. M.; Hartmann, D.; Rudolph, M.; Rominger, F.; Hashmi, A. S. K. Eur. J. Org. Chem. 2020, 2020, 1160. |
| [1] | (n) Li, Z. Y.; Wang, N. N.; Liu, J.; Mei, H. B.; Soloshonok, V. A.; Han, J. L. Org. Lett. 2021, 23, 6941. |
| [1] | (o) Xie, L. G.; Shaaban, S.; Chen, X. Y.; Maulide, N. Angew. Chem., Int. Ed. 2016, 55, 12864. |
| [1] | (p) Liu, Q. S.; Wang, L. L.; Yue, H. L.; Li, J. S.; Luo, Z. D.; Wei, W. Green Chem. 2019, 21, 1609. |
| [1] | (q) Sun, J. B.; Miao, T.; Li, P. H.; Wang, L. Chin. J. Org. Chem. 2021, 41, 3144. (in Chinese) |
| [1] | (孙佳兵, 苗涛, 李品华, 王磊, 有机化学, 2021, 41, 3144.) |
| [2] | (a) Zheng, K.; Yin, C. K.; Liu, X. H.; Lin, L. L.; Feng, X. M. Angew. Chem. Int. Ed. 2011, 50, 2573. |
| [2] | (b) Pastor, M.; Vayer, M.; Weinstabl, H.; Maulide, N. J. Org. Chem. 2022, 87, 606. |
| [2] | (c) Gambacorta, G.; Apperley, D. C.; Baxendale, I. R. Molecules 2020, 25, 2160. |
| [2] | (d) El-Hussieny, M.; El-Sayed, N. F.; Fouad, M. A.; Ewies, E. F. Bioorg. Chem. 2021, 117, 105421. |
| [2] | (e) Han, J. S.; Zhang, J. L.; Zhang, W. Q.; Gao, Z. W.; Xu, L. W.; Jian, Y. J. J. Org. Chem. 2019, 84, 7642. |
| [3] | (a) Mo, Y. Z.; Zhao, J. P.; Chen, W. P.; Wang, Q. F. Res. Chem. Intermediat. 2015, 41, 5869. |
| [3] | (b) Griffiths, B. M.; Burl, J. D.; Wang, X. Synlett 2016, 27, 2039. |
| [3] | (c) Ori, M.; Toda, N.; Takami, K.; Tago, K.; Kogen, H. Tetrahedron 2005, 61, 2075. |
| [3] | (d) Boominathan, S. S. K.; Wang, J. J. Chem.-Eur. J. 2015, 21, 17044. |
| [3] | (e) Singh, S.; Gajulapati, V.; Gajulapati, K.; Goo, J. I.; Park, Y. H.; Jung, H. Y.; Lee, S. Y.; Choi, J. H.; Kim, Y. K.; Lee, K.; Heo, T. H.; Choi, Y. Bioorg. Med. Chem. Lett. 2016, 26, 1282. |
| [3] | (f)Wang, W.; Cencic,R.; L. Whitesell, J. Pelletier, J.; Porco, J. A. Wang, W.; Cencic, R.; L. Whitesell, J. Pelletier, J.; Porco, J. A. Chem.-Eur. J. 2016, 22, 12006 |
| [3] | (h) Franke, J.; Kim, J.; Hamilton, J. P.; Zhao, D. Y.; Pham, G. M., Wiegert-Rininger, K.; Crisovan, E.; Newton, L.; Vaillancourt, B.; Tatsis, E.; Buell, C. R.; O'Connor, S. E. ChemBioChem 2019, 20, 83. |
| [3] | (i) Khuzhaev, V. U.; Zhalolov, I.; Turgunov, K. K.; Tashkhodzhaev, B.; Levkovich, M. G.; Aripova, S. F.; Shashkov, A. S. Chem. Nat. Compd. 2004, 40, 269. |
| [4] | (a) De Silva, N. H.; Dahdah, A.; Blanch, E. W.; Huegel, H. M.; Maniam, S. Eur. J. Med. Chem. 2022, 240. |
| [4] | (b) Kinthada, L. K.; Ghosh, S.; Babu, K. N.; Sharique, M.; Biswas, S.; Bisai, A. Org. Biomol. Chem. 2014, 12, 8152. |
| [4] | (c) Seo, D. Y.; Min, B. K.; Roh, H. J.; Kim, J. N. B. Korean Chem. Soc. 2017, 38, 1231. |
| [4] | (d) Chandran, R.; Pise, A.; Shah, S. K.; Rahul, D.; Baluni, A.; Tiwari, K. N. Synth. Commun. 2021, 51, 245. |
| [4] | (e) Sohail, M.; Tanaka, F. Chem.-Eur. J. 2020, 26, 222. |
| [5] | (a) Kamano, Y.; Zhang, H. P.; Ichihara, Y.; Kizu, H.; Komiyama, K.; Pettit, G. R. Tetrahedron Lett. 1995, 36, 2783. |
| [5] | (b) Rasmussen, H. B.; MacLeod, J. K. J. Nat. Prod. 1997, 60, 1152. |
| [5] | (c) Suchy, M.; Kutschy, P.; Monde, K.; Goto, H.; Harada, N.; Takasugi, M.; Dzurilla, M.; Balentova, E. J. Org. Chem. 2001, 66, 3940. |
| [5] | (d) Tang, Y. Q.; Sattler, I.; Thiericke, R.; Grabley, S.; Feng, X. Z. Eur. J. Org. Chem. 2001, 2001, 261. |
| [6] | (a) Kumar, G. S.; Ramesh, P.; Kumar, A. S.; Swetha, A.; Meshram, H. M. Tetrahedron Lett. 2013, 54, 5048. |
| [6] | (b) Zhou, Z. Q.; Xu, Y.; Zhu, B. Y.; Li, P.; Hu, G. W.; Yang, F.; Xu, S. J.; Zhang, X. X. New J. Chem. 2020, 44, 20303. |
| [6] | (c) Liu, D. Y.; Zhao, G. W.; Xiang, L. Eur. J. Org. Chem. 2010, 2010, 3975. |
| [6] | (d) Wang, D. S.; Chen, Q. A.; Lu, S. M.; Zhou, Y. G. Chem. Rev. 2012, 112, 2557. |
| [6] | (e) Repka, L. M.; Reisman, S. E. J. Org. Chem. 2013, 78, 12314. |
| [6] | (f) Zhuo, C. X.; Zheng, C.; You, S. L. Acc. Chem. Res. 2014, 47, 2558. |
| [6] | (g) Zi, W. W.; Zuo, Z. W.; Ma, D. W. Acc. Chem. Res. 2015, 48, 702. |
| [6] | (h) Roche, S. P.; Tendoung, J. J. Y.; Treguier, B. Tetrahedron 2015, 71, 3549. |
| [6] | (i) Wang, Y. S.; Xie, F. K.; Lin, B.; Cheng, M. S.; Liu, Y. X. Chem.- Eur. J. 2018, 24, 14302. |
| [6] | (j) Huang, G. H.; Yin, B. L. Adv. Synth. Catal. 2019, 361, 405. |
| [6] | (k) Silva, T. S.; Rodrigues, M. T.; Santos, H.; Zeoly, L. A.; Almeida, W. P.; Barcelos, R. C.; Gomes, R. C.; Fernandes, F. S.; Coelho, F. Tetrahedron 2019, 75, 2063. |
| [7] | (a) Peris, G.; Vedejs, E. J. Org. Chem. 2015, 80, 3050. |
| [7] | (b) Nakazaki, A.; Mori, A.; Kobayashi, S.; Nishikawa, T. Tetrahedron Lett. 2012, 53, 7131. |
| [7] | (c) Ulikowski, A.; Furman, B. Org. Lett. 2016, 18, 149. |
| [7] | (d) Tokunaga, T.; Hume, W. E.; Nagamine, J.; Kawamura, T.; Taiji, M.; Nagata, R. Bioorg. Med. Chem. Lett. 2005, 15, 1789. |
| [8] | (a) Nasim, A.; Thomas, G. T.; Ovens, J. S.; Newman, S. G. Org. Lett. 2022, 24, 7232. |
| [8] | (b) Kise, N.; Sasaki, K.; Sakurai, T. Tetrahedron, 2014, 70, 9668. |
| [8] | (c) Mandal, T.; Jana, S.; Dash, J. Eur. J. Org. Chem. 2017, 2017, 4972. |
| [8] | (d) Reddy, G. S.; Hossain, K. A.; Kumar, J. S.; Thirupataiah, B.; Edwin, R. K.; Giliyaru, V. B.; Hariharapura, R. C.; Shenoy, G. G.; Misra, P.; Pal, M. RSC Adv. 2020, 10, 289. |
| [9] | (a) Kaur, J.; Kumar, A.; Chimni, S. S., Tetrahedron Lett. 2014, 55, 2138. |
| [9] | (b) Hanhan, N. V.; Sahin, A. H.; Chang, T. W.; Fettinger, J. C.; Franz, A. K. Angew. Chem. Int. Ed. 2010, 49, 744. |
| [9] | (c) Ramachary, D. B.; Reddy, G. B.; Mondal, R. Tetrahedron Lett. 2007, 48, 7618. |
| [9] | (d) Nicolaou, K. C.; Rao, P. B.; Hao, J. L.; Reddy, M. V.; Rassias, G.; Huang, X. H.; Chen, D. Y. K.; Snyder, S. A. Angew. Chem., Int. Ed. 2003, 42, 1753. |
| [9] | (e) Zhang, J. W.; Wu, H.; Zhang, W. X.; Wang, L. M.; Jin, Y. Chin. J. Org. Chem. 2021, 41, 1187. (in Chinese) |
| [9] | (张俊伟, 吴昊, 张伟鑫, 王黎明, 金瑛, 有机化学, 2021, 41, 1187.) |
| [10] | (a) Wei, W. T.; Zhu, W. M.; Shao, Q. J.; Bao, W. H.; Chen, W. T.; Chen, G. P.; Luo, Y. J.; Liang, H. Z. ACS Sustainable Chem. Eng. 2018, 6, 8029. |
| [10] | (b) Ohmatsu, K.; Ando, Y.; Ooi, T. Synlett 2017, 28, 1291. |
| [10] | (c) Ying, W. W.; Zhu, W. M.; Gao, Z. H.; Liang, H. Z.; Wei, W. T. Synlett 2018, 29, 663. |
| [11] | (a) Shin, I.; Ramgren, S. D.; Krische, M. J. Tetrahedron 2015, 71, 5776. |
| [11] | (b) Shirai, T.; Yamamoto, Y. Organometallics 2015, 34, 3459. |
| [11] | (c) Shirai, T.; Ito, H.; Yamamoto, Y. Angew. Chem., Int. Ed. 2014, 53, 2658. |
| [11] | (d) He, J. Q.; Chen, C.; Yu, W. B.; Liu, R. R.; Xu, M.; Li, Y. J.; Gao, J. R.; Jia, Y. X. Tetrahedron Lett. 2014, 55, 2805. |
| [11] | (e) Li, Y.; Zhu, D. X.; Xu, M. H. Chem. Commun. 2013, 49, 11659. |
| [11] | (f) Hu, J. X.; Wu, H.; Li, C. Y.; Sheng, W. J.; Jia, Y. X.; Gao, J. R. Chem.-Eur. J. 2011, 17, 5234. |
| [11] | (g) Gorokhovik, I.; Neuville, L.; Zhu, J. P. Org. Lett. 2011, 13, 5536. |
| [11] | (h) Jia, Y. X.; Katayev, D.; Kundig, E. P. Chem. Commun. 2010, 46, 130. |
| [12] | Zhao, Y. W.; Zhu, H. R.; Sung, S. Y.; Wink, D. J.; Zadrozny, J. M.; Driver, T. G. Angew. Chem., Int. Ed. 2021, 60, 19207. |
| [13] | Aksenov, A. V.; Aleksandrova, E. V.; Aksenov, D. A.; Aksenova, A. A.; Aksenov, N. A.; Nobi, M. A.; Rubin, M. J. Org. Chem. 2022, 87, 1434. |
| [14] | (a) Fan, H.; Xu, Y.; Yang, F.; Xu, S. J.; Zhao, X. C.; Zhang, X. X. Adv. Synth. Catal. 2022, 364, 2358. |
| [14] | (b) Xu, Y.; Fan, H.; Yang, F.; Xu, S. J.; Zhao, X. C.; Liao, X. M.; Zhang, X. X. J. Org. Chem. 2023, 88, 2801. |
| [15] | Mei, H.; Wang, N.; Li, Z.; Han, J. Org. Lett. 2022, 24, 2258. |
| [16] | Wetzel, A.; Gagosz, F. Angew. Chem., Int. Ed. 2011, 50, 7354. |
| [17] | Bumagin, N. A.; Sukhomlinova, L. I.; Luzikova, E. V.; Tolstaya, T. P.; Beletskaya, I. P. Tetrahedron Lett. 1996, 37, 897. |
| [18] | Xiao, Z. K.; Yin, H. Y.; Shao, L. X. Org. Lett. 2013, 15, 1254. |
| [19] | Chaudhari, M. B.; Sutar, Y.; Malpathak, S.; Hazra, A.; Gnana- prakasam, B. Org. Lett. 2017, 19, 3628. |
/
| 〈 |
|
〉 |