

四丁基碘化胺介导烷基酰胺与酰肼一锅法构建1,3,4-噁二唑衍生物
收稿日期: 2023-04-04
修回日期: 2023-06-02
网络出版日期: 2023-07-06
基金资助
国家自然科学基金(21961038); 上海合作组织科技伙伴计划(2022E01049)
Tetrabutylammonium Iodide-Mediated One-Pot Construction of 1,3,4-Oxadiazole Derivatives with Alkyl Amide and Hydrazine
Received date: 2023-04-04
Revised date: 2023-06-02
Online published: 2023-07-06
Supported by
National Natural Science Foundation of China(21961038); Shanghai Cooperation Organization Science and Technology Partnership Program(2022E01049)
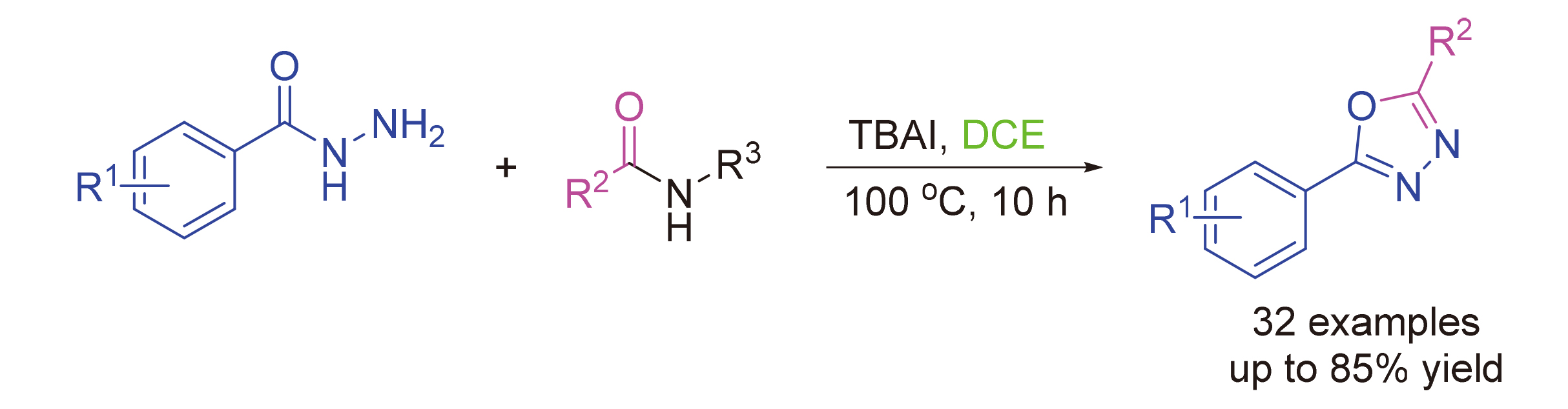
1,3,4-噁二唑衍生物是一类重要的生物活性分子, 表现出良好的抗菌、抗炎和抗癌活性. 该研究发展了一种无碱、无金属条件下, 以酰肼和烷基酰胺为原料, 一锅法构建五元杂环噁二唑的有效合成方法. 反应10 h, 即可以高达85%的收率得到预期的1,3,4-噁二唑衍生物. 该方法具有操作简单、条件温和、副产物少和底物范围广等优点. 并且可以实现克级制备, 展示出良好的实用价值. 机理研究表明酰胺自身电子云的互变异构和四丁基碘化胺(TBAI)以及酰肼羰基的活性作用对反应的发生起到了至关重要的作用.
关键词: 1,3,4-噁二唑; 烷基酰胺; 芳基甲酰肼; 四丁基碘化胺(TBAI); 一锅法
景智霞 , 杜建喜 , 蒋平 , 阿布拉江?克依木 . 四丁基碘化胺介导烷基酰胺与酰肼一锅法构建1,3,4-噁二唑衍生物[J]. 有机化学, 2023 , 43(11) : 3930 -3938 . DOI: 10.6023/cjoc202304005
1,3,4-Oxadiazole derivatives are a class of important bioactive molecules, showing good antibacterial, anti-inflam- matory and anticancer activities. In the present study, an efficient one-pot synthetic methodology has been developed for the construction of 1,3,4-oxadiazoles by utilizing acylhydrazine and alkylamide as reactants in the absence of alkaline or metallic catalysts. The expected 1,3,4-oxadiazole derivatives can be obtained with a maximum 85% yield for 10 h. This method has the advantages of simple operation, mild conditions, few by-products and wide range of substrates. And it can achieve gram preparation, showing good practical value. The mechanism study showed that the tautomerism of amide electron cloud, and the activity of tetrabutylammonium iodide (TBAI) and hydrazide carbonyl group played an important role in the reaction.

| [1] | (a) Fan, Y.; He, Y.; Liu, X.; Hu, T.; Ma, H.; Yang, X.; Luo, X.; Huang, G. J. Org. Chem. 2016, 81, 6820. |
| [1] | (b) Bozorov, K.; Nie, L. F.; Zhao, J.; Aisa, H. A. Eur. J. Med. Chem. 2017, 140, 465. |
| [1] | (c) Liu, S.; Zhao, Z.; Wang, Y. Chem.-Eur. J. 2019, 25, 2423. |
| [1] | (d) Bostrom, J.; Hogner, A.; Llinas, A.; Wellner, E.; Plowright, A. T. J. Med. Chem. 2012, 55, 1817. |
| [2] | (a) Aksenov, A. V.; Khamraev, V.; Aksenov, N. A.; Kirilov, N. K.; Domenyuk, D. A.; Zelensky, V. A.; Rubin, M. RSC Adv. 2019, 9, 6636. |
| [2] | (b) Abd-Ellah, H. S.; Abdel-Aziz, M.; Shoman, M. E.; Beshr, E. A.; Kaoud, T. S.; Ahmed, A. F. Bioorg. Chem. 2016, 69, 48. |
| [2] | (c) Boudreau, M. A.; Ding, D.; Meisel, J. E.; Janardhanan, J.; Spink, E.; Peng, Z.; Qian, Y.; Yamaguchi, T.; Testero, S. A.; O'Daniel, P. I.; Leemans, E.; Lastochkin, E.; Song, W.; Schroeder, V. A.; Wolter, W. R.; Suckow, M. A.; Mobashery, S.; Chang, M. ACS Med. Chem. Lett. 2020, 11, 322. |
| [2] | (d) Padejjar Vasantha, S.; Poojary, B.; Bistuvalli Chandrashekarappa, R. J. Chin. Chem. Soc. 2019, 66, 638. |
| [2] | (e) Wang, P. Y.; Zhou, L.; Zhou, J.; Wu, Z. B.; Xue, W.; Song, B. A.; Yang, S. Bioorg. Med. Chem. Lett. 2016, 26, 1214. |
| [2] | (f) Valente, S.; Trisciuoglio, D.; Luca, T. D.; Nebbioso, A.; Labella, D.; Lenoci, A.; Bigogno, C.; Dondio, G.; Miceli, M.; Brosch, G.; Bufalo, D. D.; Altucci, L.; Mai, A. J. Med. Chem. 2014, 57, 6259. |
| [3] | (a) Najare, M. S.; Patil, M. K.; Nadaf, A. A.; Mantur, S.; Garbhagudi, M.; Gaonkar, S.; Inamdar, S. R.; Khazi, I. A. M. J. Mol. Struct. 2020, 1199. |
| [3] | (b) Liu, Y.; Xing, K. Q.; Deng, J. Y.; Zhu, M. X.; Wang, X. Y.; Zhu, W. G. Chin. Chem. Lett. 2007, 18, 573. |
| [4] | (a) Weng, C.; Liu, Z.; Guo, H.; Tan, S. Macromol. Chem. Phys. 2017, 218, 170094. |
| [4] | (b) Chwarzer, K.; Tullmann, C. P.; Grassl, S.; Gorski, B.; Brocklehurst, C. E.; Knochel, P. Org. Lett. 2020, 22, 1899. |
| [4] | (c) Hou, R. B.; Su, J. Y.; Zhang, L. L.; Li, D. F.; Xia, Y. J. Chem. Res. 2019, 43, 3. |
| [5] | (a) Stabile, P.; Lamonica, A.; Ribecai, A.; Castoldi, D.; Guercio, G.; Curcuruto, O. Tetrahedron Lett. 2010, 51, 4801. |
| [5] | (b) Li, M. F.; Wang, R.; Hao, W. J.; Jiang, B. Chin. J. Org. Chem. 2020, 40, 1540. (in Chinese) |
| [5] | (李梦帆, 王榕, 郝文娟, 姜波, 有机化学, 2020, 40, 1540.) |
| [5] | (c) Zhang, X.; He, J.; Cao, S. Asian J. Org. Chem. 2019, 8, 279. |
| [5] | (d) Fugard, A. J.; Thompson, B. K.; Slawin, A. M.; Taylor, J. E.; Smith, A. D. Org. Lett. 2015, 17, 5824. |
| [5] | (e) Green, L.; Livingstone, K.; Bertrand, S.; Peace, S.; Jamieson, C. Chem.-Eur. J. 2020, 26, 14866. |
| [6] | (a) Gao, Q.; Liu, S.; Wu, X.; Zhang, J.; Wu, A. Org. Lett. 2015, 17, 2960. |
| [6] | (b) Shivi, B.; Monika, G. J. Chem. Pharm. Res. 2011, 3, 137. |
| [7] | Li, Q.; Tao, Y.; Xu, D.; Zhang, H.; Duan, L. J. Chin. Chem. Soc. 2014, 61, 665. |
| [8] | (a) Niu, P.; Kang, J.; Tian, X.; Song, L.; Liu, H.; Wu, J.; Yu, W.; Chang, J. J. Org. Chem. 2015, 80, 1018. |
| [8] | (b) Chauhan, J.; Ravva, M. K.; Sen, S. Org. Lett. 2019, 21, 6562. |
| [9] | Wang, Q.; Wang, X.; Liu, Q.; Xie, G.; Ding, S.; Wang, X.; Fan, H. Org. Chem. Front. 2020, 7, 3912. |
| [10] | Fugard, A. J.; Thompson, B. K.; Slawin, A. M.; Taylor, J. E.; Smith, A. D. Org. Lett. 2015, 17, 5824. |
| [11] | Lu, F.; Gong, F.; Li, L. Eur. J. Org. Chem. 2020, 3257. |
| [12] | (a) Li, A. F.; Ruan, Y. B.; Jiang, Q. Q.; He, W. B.; Jiang, Y. B. Chem.-Eur. J. 2010, 16, 5794. |
| [12] | (b) Pouliot, M. F.; Angers, L.; Hamel, J. D.; Paquin, J. F. Org. Biomol. Chem. 2012, 10, 988. |
| [12] | (c) Chauhan, J.; Ravva, M. K.; Sen, S. Org. Lett. 2019, 21, 6562. |
| [13] | Yu, W.; Huang, G.; Zhang, Y. T.; Liu, H. X.; Dong, L. H.; Yu, X. J.; Li, Y. J.; Chang, J. B. J. Org. Chem. 2013, 78, 10337. |
| [14] | Wang, L.; Wang, Y. Y; Chen, Q.; He, M. Y. Tetrahedron Lett. 2018, 59, 1489. |
| [15] | Wang, Q.; Wang, X.; Liu, Q.; Xie, G.; Ding, S.; Wang, X. X; Fan, H. Org. Chem. Front. 2020, 7, 3912. |
| [16] | Shu, W. M.; Zhang, X. Zhang, X. X.; Li, M.; Wang, A. J.; Wu, A. X. J. Org. Chem. 2019, 84, 14919. |
| [17] | Wang, S.; Wang, K.; Kong, X.; Zhang, S.; Jiang, G.; Ji, F. Adv. Synth. Catal. 2019, 361, 3986. |
| [18] | (a) Wang, Q.; Mgimpatsang, K. C.; Konstantinidou, M.; Shishkina, S. V.; Do?mling, A. Org. Lett. 2019, 21, 7320. |
| [18] | (b) Zhang, L.; Yu, Y.; Tang, Q.; Yuan, J.; Ran, D.; Tian, B.; Pan, T.; Gan, Z. Synth. Commun. 2019, 50, 423. |
| [19] | (a) Suresh, D.; Kanagaraj, K.; Pitchumani, K. Tetrahedron Lett. 2014, 55, 3678. |
| [19] | (b) Wang, Y.; Meng, Xu.; Yang, Y. T.; Chen, B. H. Chem. Commun. 2015, 51, 1907. |
| [20] | Majji, G.; Rout, S. K.; Guin, S.; Gogoi, A.; Patel, B. K. RSC Adv. 2014, 4, 5357. |
| [21] | Su, X. L.; Ye, L.; Chen, J. J.; Liu, X. D.; Jiang, S. P.; Jiang, F. L.; Liu, X. Y. Angew. Chem., Int. Ed. 2021, 60, 380. |
| [22] | Zou, L. H.; Reball, J.; Mottweiler, J.; Bolm, C. Chem. Commun. 2012, 48, 11307. |
| [23] | (a) Zhang, Q. W.; Wang, B.; Ma, H. F.; Ablajan, K. New J. Chem. 2019, 43, 17000. |
| [23] | (b) Jia, Y. F.; Ablajan, K. Adv. Synth. Catal. 2023, 365, 244. |
| [23] | (c) Liang, J.; Ma, H. F.; Ablajan, K. Chin. J. Org. Chem. 2019, 39, 3169. |
| [24] | (a) Siwach, A.; Verma, P. K. BMC Chem. 2020, 14, 70. |
| [24] | (b) Gao, P.; Wang, J.; Bai, Z.; Cheng, H.; Xiao, J.; Lai, M.; Yang, D.; Fan, M. Tetrahedron Lett. 2016, 57, 4616. |
| [24] | (c) Dong, D. Q.; Zhang, H.; Wang, Z. L. RSC Adv. 2017, 7, 3780. |
| [24] | (d) Li, L. X.; Dong, D. Q.; Hao, S. H.; Wang, Z. L. Tetrahedron Lett. 2018, 59, 1517. |
| [25] | Zahra, D. G.; Karim, A. D. J. Chin. Chem. Soc. 2020, 67, 1446. |
| [26] | Gnanasekaran, K. K.; Nammalwar, B.; Murie, M.; Bunce, R. A. Tetrahedron Lett. 2014, 55, 6776. |
| [27] | Polshettiwar, V.; Varma, R. S. Tetrahedron Lett. 2008, 49, 879. |
/
| 〈 |
|
〉 |