

钯催化3-(2-氨基嘧啶-4-基)吲哚2位C—H键芳基化反应的研究
收稿日期: 2023-04-09
修回日期: 2023-05-24
网络出版日期: 2023-07-06
基金资助
黑龙江省自然科学基金(LH2022B003); 高等学校学科创新引智计划(111计划)(B20088)
Pd-Catalyzed C(2)—H Arylation of 3-(2-Aminopyrimidin-4-yl)indoles
Received date: 2023-04-09
Revised date: 2023-05-24
Online published: 2023-07-06
Supported by
Natural Science Foundation of Heilongjiang Province(LH2022B003); the Programme of Introducing Talents of Discipline to Universities (111 Project)(B20088)
孙美娇 , 谭晶 , 谭玉 , 彭进松 , 陈春霞 . 钯催化3-(2-氨基嘧啶-4-基)吲哚2位C—H键芳基化反应的研究[J]. 有机化学, 2023 , 43(11) : 3945 -3959 . DOI: 10.6023/cjoc202304012
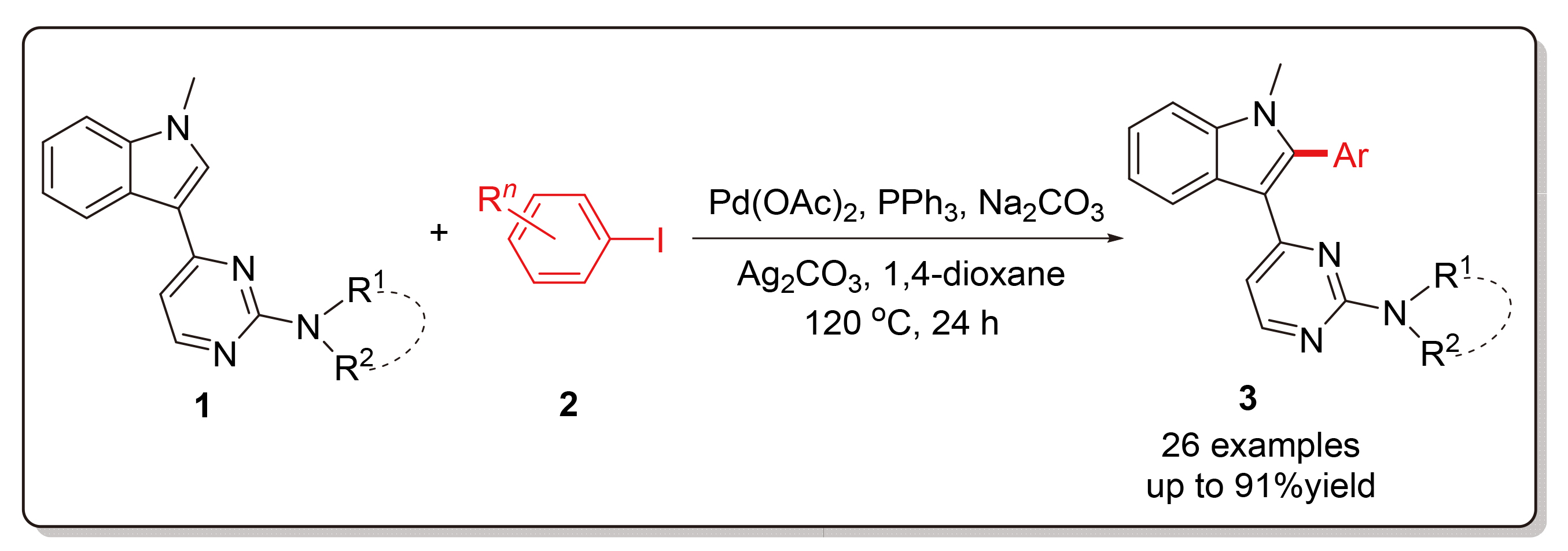
Using N-methyl-3-(2-aminopyrimidin-4-yl)indole derivatives and aryl iodides as starting materials, palladium- catalyzed selective C—H bond activation/arylation at the C-2 position of indole was investigated in detail. With palladium acetate as the catalyst, triphenylphosphine as the ligand, silver carbonate as the additive and sodium carbonate as the base, various 2-arylindole products were obtained in high yields in 1,4-dioxane at 120 ℃ for 24 h. Under these standard conditions, the scope and limitation of the synthetic method were explored by changing the structure of two substrates, and the synthesized indole derivatives were characterized by 1H NMR, 13C NMR and HRMS spectra.
| [1] | (a) Dorababu, A. RSC Med. Chem. 2020, 11, 1335. |
| [1] | (b) Han, Y.; Dong, W.; Guo, Q.; Li, X; Huang, L. Eur. J. Med. Chem. 2020, 203, 112506. |
| [1] | (c) Qin, H. L.; Liu, J.; Fang, W. Y.; Ravindar, L; Rakesh, K. P. Eur. J. Med. Chem. 2020, 194, 112245. |
| [1] | (d) Colenda, B. E.; Lee, H. S.; Reibenspies, J. H.; Hancock, R. D. Inorg. Chim. Acta. 2018, 482, 478. |
| [1] | (e) Zhao, X. H.; Jia, Y. X.; Li, J. J.; Dong, R.H.; Zhang, J.J.; Ma, C. X.; Wang, H.; Rui, Y.K.; Jiang, X.Y. ACS Appl. Mater. Inter. 2018, 35, 29398. |
| [1] | (f) Mohbiya, D. R.; Sekar, N. Comput. Theor. Chem. 2018, 1139, 90. |
| [1] | (g) Zhao, J. F.; Dong, H.; Zheng, Y. J. J. Lumin. 2018, 195, 228. |
| [2] | Zhang, M. Z.; Chen, Q.; Yang, G. F. Eur. J. Med. Chem. 2015, 89, 42. |
| [3] | (a) Austin, O.; Chelsea, S.; Patrick, S.; Kent, A. C. ACS Med. Chem. Lett. 2018, 9, 901. |
| [3] | (b) Cong, H.; Zhao, X. H.; Castle, B. T.; Pomeroy, E. J.; Zhou, B.; John, L.; Wang, Y.; Bian, T. F.; Miao, Z. Y.; Zhang, W. N.; Sham, Y. Y.; David, J. O.; Craig, E. E.; Xing, C. G.; Zhuang, C. L. Mol. Pharmaceut. 2018, 15, 3892. |
| [3] | (c) Singh, P. K.; Silakari, O. Bioorg. Chem. 2018, 79, 163. |
| [4] | Kaushik, N. K.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C. H.; Verma, A. K.; Choi, E. H. Molecules 2013, 18, 6620. |
| [5] | Ding, C. F.; Ma, H. X.; Yang, J.; Qin, X. J.; Guy, S. S.; Yu, H. F.; Wei, X.; Liu, Y. P.; Huang, W. Y.; Yang, Z. F.; Wang, X. H.; Luo, X. D.; Org. Lett. 2018, 20, 2702. |
| [6] | (a) Zhang, Z.; Tanaka, K.; Yu, J. Q. Nature. 2017, 543, 538. |
| [6] | (b) Zhang, F. L.; Hong, K.; Li, T. J.; Yu, J. Q. Science 2016, 351, 252. |
| [7] | Dalpozzo, R.; Bartoli, G.; Bencivenni, G. Chem. Soc. Rev. 2012, 41, 7247. |
| [8] | Kumari, A.; Singh, R. K. Bioorg. Chem. 2019, 89, 103021. |
| [9] | (a) Zhang, S. S.; Tan, Q. W.; Guan, L. P. Mini-Rev. Med. Chem. 2021, 21, 2285. |
| [9] | (b) Okada, M.; Sugita, T.; Wong, C. P.; Wakimoto, T.; Abe, I. J. Nat. Prod. 2017, 80, 1205. |
| [9] | (c) Hong, W.; Li, J.; Chang, Z; Tan, X. L.; Yang, Y.; Ouyang, Y. F.; Yang, Y. H.; Kaur, S.; Paterson, I. C.; Ngeow, Y. F.; Wang, H. J. Antibiot. 2017, 70, 832. |
| [9] | (d) Kherkhache, H.; Benabdelaziz, I.; Silva, A. M. S.; Lahrech, M. B.; Benalia, M.; Haba, H. Nat. Prod. Res. 2020, 34, 1528. |
| [9] | (e) Pulla, R. S.; Ummadi, N.; Gudi, Y.; Venkatapuram, P.; Adivireddy, P. J. Heterocyclic. Chem. 2018, 55, 115. |
| [10] | (a) Esvan, Y. J.; Giraud, F.; Pereira, E.; Suchaud, V.; Nauton, L.; Théry, V.; Dezhenkova, L. G.; Kaluzhny, D. N.; Mazov, V. N.; Shtil, A. A.; Anizon, F.; Moreau, P. Bioorg. Med. Chem. 2016, 24, 3116. |
| [10] | (b) Karimabad, M. N.; Mahmoodi, M.; Jafarzadeh, A.; Darekordi, A.; Hajizadeh, M. R.; Hassanshahi, G. Mini-Rev. Med. Chem. 2019, 19, 540. |
| [11] | Bian, M.; Wang, Z.; Xiong, X.; Sun, Y.; Matera, C.; Nicolaou, K. C.; Li, A. J. Am. Chem. Soc. 2012, 134, 8078. |
| [12] | Vasconcelos, S. N.; Meissner, K. A.; Ferraz, W. R.; Trossini, G. H.; Wrenger, C.; Stefani, H. A. Future Med. Chem. 2019, 11, 525. |
| [13] | Chadha, N.; Silakari, O. Eur. J. Med. Chem. 2017, 134, 159. |
| [14] | Singh, P.; Prasher, P.; Dhillon, P.; Bhatti, R. Eur. J. Med. Chem. 2015, 97, 104. |
| [15] | Sravanthi, T. V.; Manju, S. L. Eur. J. Pharm. Sci. 2016, 91, 1. |
| [16] | Ye, Q. Y.; Xu, Y.; Zhao, J.; Gao, X. X; Chen, M. J.; Pan, R. l.; Zhong, W.; Wang, M. Z. Transl. Oncol. 2023, 31, 101637. |
| [17] | Barnes, L.; Blaber, H.; Brooks, D. T. K.; Byers, L.; Buckley, D.; Byron, Z. C.; Chilvers, R. G.; Cochrane, L.; Cooney, E.; Damian, H. A.; Francis, L.; He, D. F.; Grace, J. M. J.; Green, H. J.; Hogarth, E. J. P.; Jusu, L.; Killalea, C. E.; King, O.; Lambert, J.; Lee, Z. J.; Lima, N. S.; Long, C. L.; Mackinnon, M.-L.; Mahdy, S.; Matthews-Wright, J.; Millward, M. J.; Meehan, M. F.; Merrett, C.; Morrison, L.; Parke, H. R. I.; Payne, C.; Payne, L.; Pike, C.; Seal, A.; Senior, A. J.; Smith, K. M.; Stanelyte, K.; Stillibrand, J.; Szpara, R.; Taday, F. F. H.; Threadgould, A. M.; Trainor, R. J.; Waters, J.; Williams, O.; Wong, C. K. W.; Wood, K.; Barton, N.; Gruszka, A.; Henley, Z.; Rowedder, J. E.; Cookson, R.; Jones, K. L.; Nadin, A.; Smith, I. E.; Macdonald, S. J. F.; Nortcliffe, A. J. Med. Chem. 2019, 62, 10402. |
| [18] | (a) Jian, H. H.; Xin, R. W.; Rui, N. D.; Xiao, Y. L.; Hong, M. L.; Tian, Y. Z.; Jun, Y. X.; Chen, H. L.; Yan, M.; Zhang, S. H.; Hou, W. F.; Tang, T. L.; Ya, D. C. J. Med. Chem. 2021, 64, 12548. |
| [18] | (b) Yuan, S.; Wang, B.; Dai, Q. Q.; Zhang, X. N.; Zhang, J. Y.; Zuo, J. H.; Liu, H.; Chen, Z. S.; Li, G. B.; Wang, S. M.; Liu, H. M.; Yu, B. J. Med. Chem. 2021, 64, 14895. |
| [19] | (a) Deng, H.; Jung, J.-K.; Liu, T.; Kuntz, K. W.; Snapper, M. L.; Hoveyda, A. H. J. Am. Chem. Soc. 2003, 125, 9032. |
| [19] | (b) Nicolaou, K. C.; Bheema Rao, P.; Hao, J.; Reddy, M. V.; Rassias, G.; Huang, X.; Chen, D. Y.; Snyder, S. A. Angew. Chem., Int. Ed. 2003, 42, 1753. |
| [19] | (c) Nicolaou, K. C.; Hao, J.; Reddy, M. V.; Rao, P. B.; Rassias, G.; Snyder, S. A.; Huang, X.; Chen, D. Y. K.; Brenzovich, W. E.; Giuseppone, N.; Giannakakou, P.; O'Brate, A. J. Am. Chem. Soc. 2004, 126, 12897. |
| [19] | (d) Sitnikov, N.; Velder, J.; Abodo, L.; Cuvelier, N.; Neudorfl, J.; Prokop, A.; Krause, G.; Fedorov, A. Y.; Schmalz, H. G. Chem. - Eur. J. 2012, 18, 12096. |
| [20] | Kumar, P.; Nagtilak, P. J.; Kapur, M. New J. Chem. 2021, 45, 13692. |
| [21] | (a) Chen, Z.; Wang, B.; Zhang, J.; Yu, W.; Liu, Z.; Zhang, Y. Org. Chem. Front. 2015, 2, 1107. |
| [21] | (b) Sandtorv, A. H. Adv. Synth. Catal. 2015, 357, 2403. |
| [21] | (c) Wen, J.; Shi, Z. Acc. Chem. Res. 2021, 54, 1723. |
| [21] | (d) Luo, J. F.; Xu, X.; Zheng, J. L. Chin. J. Org. Chem. 2018, 38, 363. (in Chinese) |
| [21] | (骆钧飞, 徐星, 郑俊良, 有机化学, 2018, 38, 363.) |
| [22] | (a) Islam, S.; Larrosa, I. Chem. Eur. J. 2013, 19, 15093. |
| [22] | (b) Zheng, J.; Zhang, Y.; Cui, S. L. Org. Lett. 2014, 16, 3560. |
| [22] | (c) Miao, T.; Li, P.; Wang, G. W.; Wang, L. Chem. Asian J. 2013, 8, 3185. |
| [23] | (a) Lu, M.-Z.; Lu, P.; Xu, Y.-H.; Loh, T.-P. Org. Lett. 2014, 16, 2614. |
| [23] | (b) Lu, M.-Z.; Ding, X.; Shao, C. D.; Hu, Z. S.; Luo, H. Q.; Zhi, S. Z.; Hu, H. Y.; Kan, Y. H.; Loh, T.-P. Org. Lett. 2020, 22, 2663. |
| [23] | (c) Tiwari, V. K.; Kamal, N.; Kapur, M. Org. Lett. 2015, 17, 1766. |
| [24] | (a) Sun, P.; Yang, J. J.; MO, B. C.; Chen, X.; Li, X.; Chen, C. X. J. Org. Chem. 2020, 85, 6761. |
| [24] | (b) Chen, X.; Sun, P.; MO, B. C.; Chen, C. X.; Peng, J. S. J. Org. Chem. 2021, 86, 352. |
| [24] | (c) Li, X.; Chen, X.; Wang, H.; Chen, C. X.; Sun, P.; MO, B. C.; Peng, J. S. Org. Biomol. Chem. 2019, 17, 4014. |
| [24] | (d) Li, X.; Bian, Y. Y.; Chen, X.; Zhang, H.; Wang, W.; Ren, S. D.; Yang, X. C.; Lu, C.; Chen, C. X.; Peng, J. S. Org. Biomol. Chem, 2019, 17, 321. |
| [24] | (e) Yue, Y. X.; Peng, J. S.; Wang, D. Q.; Bian, Y. Y.; Sun, P.; Chen, C. X. J. Org. Chem. 2017, 82, 5481. |
| [25] | Yanagisawa, S.; Itami, K. Tetrahedron. 2011, 67, 4425. |
| [26] | (a) Mota, A. J.; Dedieu, A.; Bour, C.; Suffert, J. J. Am. Chem. Soc. 2005, 127, 7171. |
| [26] | (b) Garcia-Cuadrado, D.; Braga, A. A. C.; Maseras, F.; Echavarren, A. M. J. Am. Chem. Soc. 2006, 128, 6798. |
| [27] | Jia, H. J.; Wu, K. Y.; Wang, Y. X. CN 108017620 2017 [Chem. Abstr. 2017, 10, 747229] |
| [28] | Zhang, X. X.; Chen, T. P.; Zhu, G. Y. CN 113563310 2021 [Chem. Abstr. 2021, 10, 709318] |
/
| 〈 |
|
〉 |