

苯并噻唑酮类热活化延迟荧光材料的合成及其光电性能研究
收稿日期: 2023-03-15
修回日期: 2023-05-30
网络出版日期: 2023-07-13
基金资助
国家自然科学基金(U2001222); 国家自然科学基金(U22A20399); 国家自然科学基金(52003058); 国家自然科学基金(21975055); 广东省基础与应用基础研究基金(2019B1515120035); 广东省基础与应用基础研究基金(2021A1515010607)
Synthesis and Optoelectronic Studies of Thermally Activated Delayed Fluorescence Materials Based on Benzothiazolyl Ketones
Received date: 2023-03-15
Revised date: 2023-05-30
Online published: 2023-07-13
Supported by
National Natural Science Foundation of China(U2001222); National Natural Science Foundation of China(U22A20399); National Natural Science Foundation of China(52003058); National Natural Science Foundation of China(21975055); Guangdong Basic and Applied Basic Research Foundation(2019B1515120035); Guangdong Basic and Applied Basic Research Foundation(2021A1515010607)
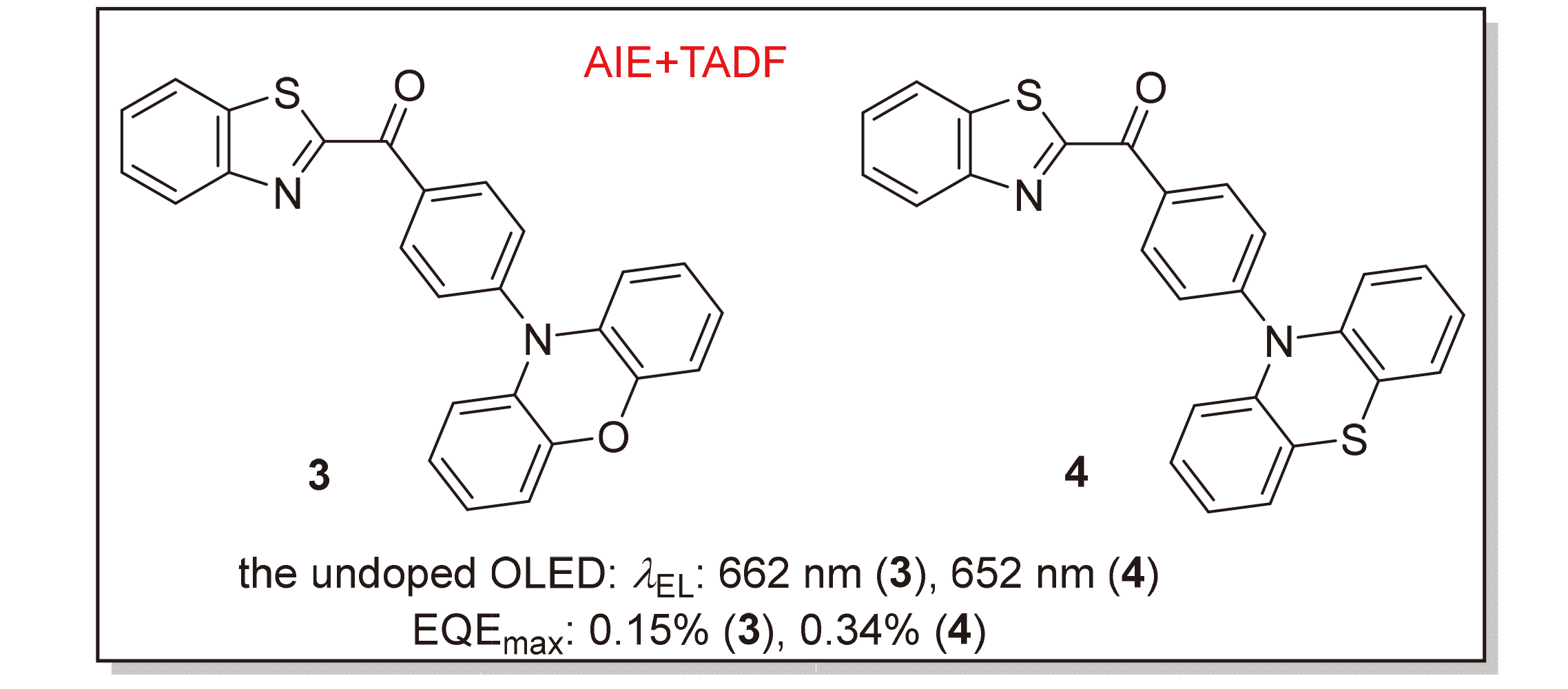
以苯并噻唑-2-基(苯基)甲酮作为受体, 具有强给电子能力的吩噁嗪和吩噻嗪作为给体构筑给体-受体(D-A)型分子, 设计合成了两种具有聚集诱导发光(AIE)特性的热活化延迟荧光(TADF)红光材料3和4, 并对它们的热稳定性、电化学性质、单晶结构、光物理性质和电致发光性能进行了系统研究. 两种化合物具有较小的单三线态能级差(ΔEST, 0.04和0.16 eV)以及微秒级延迟寿命(0.63和1.30 μs), 表现出明显的TADF特性. 通过对比化合物在粉末状态下研磨前后的发射光谱, 发现化合物4具有明显的力致变色发光现象. 在纯薄膜下, 两种化合物的发射峰分别为683和654 nm, 光致发光量子产率(PLQY)分别为0.8%和3.6%. 基于化合物3和4的非掺杂有机发光二极管(OLED)器件, 均获得了纯红光发射(662和652 nm), 器件的最大外量子效率(EQE)分别为0.15%和0.34%. 虽然基于这两种化合物的器件发光效率有待提升, 但它们的合成过程简便, 能为开发苯并噻唑酮类TADF红光材料提供一定的启发.
关键词: 聚集诱导发光(AIE)特性; 热活化延迟荧光(TADF)红光材料; 力致变色发光; 苯并噻唑酮类
张越华 , 聂飞 , 周路 , 王晓烽 , 刘源 , 霍延平 , 陈文铖 , 赵祖金 . 苯并噻唑酮类热活化延迟荧光材料的合成及其光电性能研究[J]. 有机化学, 2023 , 43(11) : 3876 -3887 . DOI: 10.6023/cjoc202303022
Two thermally activated delayed fluorescence (TADF) red-emitting materials 3 and 4 with aggregation-induced emission (AIE) properties were designed and synthesized using benzothiazole-2-yl(phenyl)methanone as acceptors, and phenoxazine and phenothiazine with strong electron-donating ability as donors to construct donor-acceptor (D-A) type molecules, and their thermal stability, electrochemical properties, single crystal structure, photophysical properties and electroluminescence properties were systematically studied. The two compounds have small singlet-triplet slitting (ΔEST, 0.04 and 0.16 eV) and microsecond-scale delayed lifetimes (0.63 and 1.30 μs), showing obvious TADF characteristics. Comparing the emission spectra before and after grinding in the powder state, it is found that compound 4 has obvious mechanochromic luminescence phenomenon. In neat-film state, the emission peaks of the two compounds were 683 and 654 nm, and photoluminescence quantum yields (PLQYs) were 0.8% and 3.6%, respectively. The non-doped organic light-emitting diode (OLED) devices based on compounds 3 and 4 obtained pure red emission (662 and 652 nm), and the maximum external quantum efficiencies (EQEs) of the devices were 0.15% and 0.34%, respectively. Although the luminous efficiencies of devices based on these two compounds are not high, their synthesis process is facile, which can provide useful insight for the development of red TADF materials based on benzothiazolyl ketones.

| [1] | Zhou, J.; Tian, X. Y.; Wang, B. K.; Zhang, S. S.; Liu, Z. H.; Chen, W. Acta Chim. Sinica 2022, 80, 395. (in Chinese) |
| [1] | (周静, 田雪迎, 王斌凯, 张沙沙, 刘宗豪, 陈炜, 化学学报, 2022, 80, 395.) |
| [2] | Liang, Z. P.; Tang, R.; Qiu, Y. C.; Wang, Y.; Lu, H.; Wu, Z. G. Acta Chim. Sinica 2021, 79, 1401. (in Chinese) |
| [2] | (梁志鹏, 唐瑞, 邱雨晨, 王阳, 陆洪彬, 吴正光, 化学学报, 2021, 79, 1401.) |
| [3] | Sun, N.; Jiang, C.; Li, Q.; Tan, D.; Bi, S.; Song, J. J. Mater. Sci.: Mater. Electron. 2020, 31, 20688. |
| [4] | Yang, X.; Jiao, B.; Dang, J. S.; Sun, Y.; Wu, Y.; Zhou, G.; Wong, W. Y. ACS Appl. Mater. Interfaces 2018, 10, 10227. |
| [5] | Wong, M. Y.; Zysman-Colman, E. Adv. Mater. 2017, 29, 1605444. |
| [6] | Tao, Y.; Yuan, K.; Chen, T.; Xu, P.; Li, H. H.; Chen, R. F.; Zheng, C.; Zhang, L.; Huang, W. Adv. Mater. 2014, 26: 7931. |
| [7] | Di, B. H.; Chen, Y. L. Chin. Chem. Lett. 2018, 29, 245. |
| [8] | Huang, C.; Qiu, Z. P.; Gao, Y.; Chen, W. C.; Ji, S. M.; Huo, Y. P. Chin. J. Org. Chem. 2021, 41, 3050. (in Chinese) |
| [8] | (黄酬, 邱志鹏, 高杨, 陈文铖, 籍少敏, 霍延平, 有机化学, 2021, 41, 3050.) |
| [9] | Tan, J. H.; Huo, Y. P.; Cai, N.; Ji, S. M.; Li, Z. Z.; Zhang, L. Chin. J. Org. Chem. 2017, 37, 2457. (in Chinese) |
| [9] | (谭继华, 霍延平, 蔡宁, 籍少敏, 李宗植, 张力, 有机化学, 2017, 37, 2457.) |
| [10] | Sarada, G.; Cho, W.; Maheshwaran, A.; Sree, V. G.; Park, H. Y.; Gal, Y. S.; Song, M.; Jin, S. H. Adv. Funct. Mater. 2017, 27, 1701002. |
| [11] | Zheng, Y. T.; Zuo, L. Q.; Zhang, L. T.; Huang, Z. H.; Li, S. F.; Yang, Z.; Mao, Z.; Luo, S. L.; Liu, C.; Sun, F. Q.; Shi, G.; Chi, Z. G.; Xu, B. J. Chin. Chem. Lett. 2022, 33, 4536. |
| [12] | Cao, H. T.; Hou, P. F.; Cao, Q.; Li, Y. A.; Wang, S. S.; Xie, L. H. Acta Chim. Sinica 2022, 80, 1476. (in Chinese) |
| [12] | (曹洪涛, 侯鹏飞, 曹庆, 李延昂, 汪莎莎, 解令海, 化学学报, 2022, 80, 1476.) |
| [13] | Shi, Q.; Wang, L. Y. Chin. J. Org. Chem. 2022, 42, 1256. (in Chinese) |
| [13] | (石强, 王乐勇, 有机化学, 2022, 42, 1256.) |
| [14] | Guo, J. J.; Zhao, Z. J.; Tang, B. Z. Adv. Opt. Mater. 2018, 6, 1800264. |
| [15] | Sagara, Y.; Shizu, K.; Tanaka, H.; Miyazaki, H.; Goushi, K.; Kaji, H.; Adachi, C. Chem. Lett. 2015, 44, 360. |
| [16] | Ahn, D. H.; Kim, S. W.; Lee, H.; Ko, I. J.; Karthik, D.; Lee, J. Y.; Kwon, J. H. Nat. Photonics 2019, 13, 540. |
| [17] | Wu, T. L.; Huang, M. J.; Lin, C. C.; Huang, P. Y.; Chou, T. Y.; Chen-Cheng, R. W.; Lin, H. W.; Liu, R. S.; Cheng, C. H. Nat. Photonics 2018, 12, 235. |
| [18] | Bryden, M. A.; Zysman-Colman, E. Chem. Soc. Rev. 2021, 50, 7587. |
| [19] | Zhang, T.; Zhou, Z.; Liu, X.; Wang, K.; Fan, Y.; Zhang, C.; Yao, J.; Yan, Y.; Zhao, Y. S. J. Am. Chem. Soc. 2021, 143, 20249. |
| [20] | Steinegger, A.; Klimant, I.; Borisov, S. M. Adv. Opt. Mater. 2017, 5, 1700372. |
| [21] | Wong, M. Y.; Zysman-Colman, E. Adv. Mater. 2017, 29, 1605444. |
| [22] | Fang, F.; Yuan, Y.; Wan, Y.; Li, J.; Song, Y.; Chen, W.; Zhao, D.; Chi, Y.; Li, M.; Lee, C. Small 2022, 18, 2106215. |
| [23] | Fang, F.; Zhu, L.; Li, M.; Song, Y. Y.; Sun, M.; Zhao, D. G.; Zhang, J. F. Adv. Sci. 2021, 8, 2102970. |
| [24] | Tan, J. M.; Yu, Y. J.; Guan, M.; Zhao, Y. H.; Tang, Z. L.; Zhou, Z. H.; Guo, T. Chin. J. Org. Chem. 2022, 42, 3776. (in Chinese) |
| [24] | (谭佳敏, 余雅君, 关猛, 赵云辉, 唐子龙, 周智华, 郭涛, 有机化学, 2022, 42, 3776.) |
| [25] | Zeng, W.; Lin, M. H.; Zhu, L. Y.; Lin, M. J. Chin. J. Chem. 2022, 40, 39. |
| [26] | Ma, H.; Peng, Q.; An, Z.; Huang, W.; Shuai, Z. J. Am. Chem. Soc. 2018, 141, 1010. |
| [27] | Ma, R.; Ding, Y.; Chen, R.; Wang, Z.; Ma, Y. J. Org. Chem. 2020, 86, 310. |
| [28] | Okazaki, M.; Takeda, Y.; Data, P.; Pander, P.; Higginbotham, H.; Monkman, A. P.; Minakata, S. Chem. Sci. 2017, 8, 2677. |
| [29] | Huang, B.; Chen, W. C.; Li, Z. J.; Zhang, J. F.; Zhao, W. J.; Feng, Y.; Tang, B. Z.; Lee, C. S. Angew. Chem., Int. Ed. 2018, 57, 12473. |
| [30] | Xu, B. J.; Mu, Y. X.; Mao, Z.; Xie, Z. L.; Wu, H. Z.; Zhang, Y.; Jin, C. J.; Chi, Z. G.; Liu, S. W.; Xu, J. R.; Wu, Y. C.; Lu, P. Y.; Lien, A.; Bryce, M. R. Chem. Sci. 2016, 7, 2201. |
| [31] | Han, M.; Chen, Y.; Xie, Y.; Zhang, F.; Li, Z. Cell Rep. Phys. Sci. 2020, 1, 100252. |
| [32] | Borowicz, P.; Herbich, J.; Kapturkiewicz, A.; Opallo, M.; Nowacki, J. Chem. Phys. 1999, 249, 49. |
| [33] | Li, S. S.; Huang, X.; Gao, Y. L.; Jin, J. Org. Lett. 2022, 24, 5817. |
| [34] | Acar, N.; Kurzawa, J.; Fritz, N.; Stockmann, A.; Roman, C.; Schneider, S.; Clark, T. J. Phys. Chem. A 2003, 107, 9530. |
| [35] | Polgar, A. M.; Poisson, J.; Paisley, N. R.; Christopherson, C. J.; Reyes, A. C.; Hudson, Z. M. Macromolecules 2020, 53, 2039. |
/
| 〈 |
|
〉 |