

可见光/路易斯碱协同催化的三氟甲基取代烯烃脱氟硅化反应研究
收稿日期: 2023-05-02
修回日期: 2023-06-30
网络出版日期: 2023-07-20
基金资助
国家自然科学基金(22171080); 上海市自然科学基金(23ZR1417200)
Research of Visible Light/Lewis Base Dual Catalytic Defluorinative Silylation of Trifluoromethyl-Substituted Alkenes
Received date: 2023-05-02
Revised date: 2023-06-30
Online published: 2023-07-20
Supported by
National Natural Science Foundation of China(22171080); Natural Science Foundation of Shanghai(23ZR1417200)
朱佳洁 , 万义 , 袁启洋 , 魏金莲 , 张永强 . 可见光/路易斯碱协同催化的三氟甲基取代烯烃脱氟硅化反应研究[J]. 有机化学, 2023 , 43(10) : 3623 -3634 . DOI: 10.6023/cjoc202304034
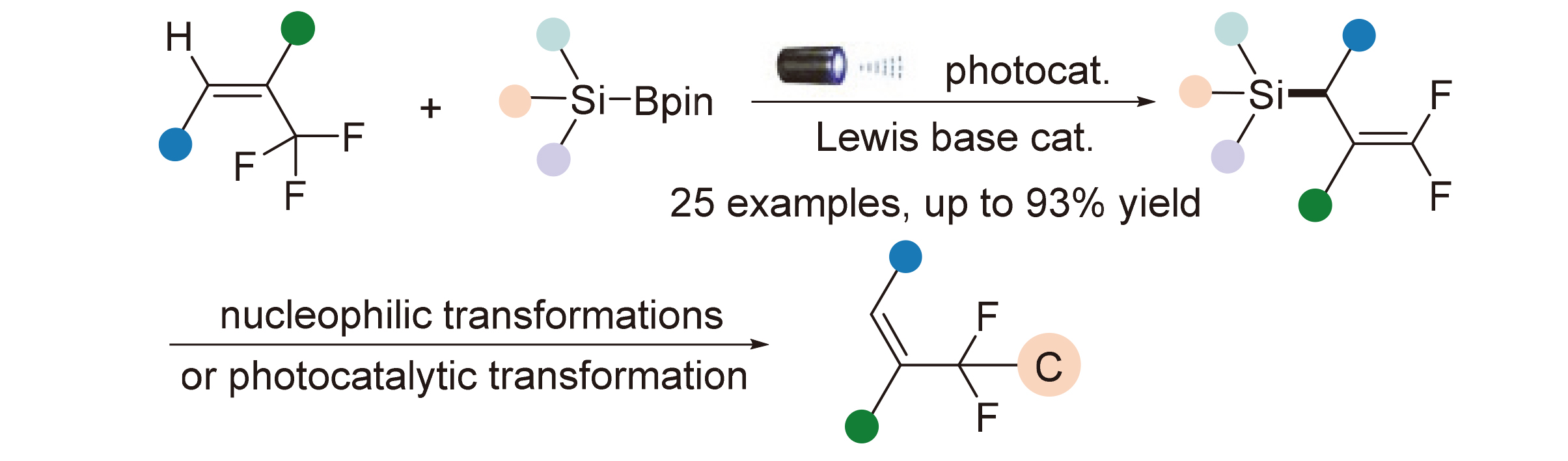
A novel photoredox/Lewis base dual-catalytic defluorinative silylation reaction of trifluoromethylsubstituted alkenes for the synthesis of gem-difluoroallylsilanes is reported, where silylboranes are employed as the silyl donor reagents. The protocol proceeds via the catalytic activation of Si—B bond by a Lewis base quinuclidine and the formation of silyl radical, making it easy to scale up. It features mild and green reaction conditions, simple reaction system, broad substrate scope and good functional group compatibility. Furthermore, the potential of this class of building blocks in the construction of various gem-difluoromethyl-containing structures has been also demostrated.
| [1] | Meanwell N. A. J. Med. Chem. 2011, 54, 2529. |
| [2] | Han J.-L.; Remete A. M.; Hagan D.; Dobson L. S.; Kiss L.; Izawa K.; Moriwaki H.; Soloshonok V. A. J. Fluorine Chem. 2020, 239, 109639. |
| [3] | Inoue M.; Sumii Y.; Shibata N. ACS Omega 2020, 5, 10633. |
| [4] | Zafrani Y.; Moriah G. S.; Yeffet D.; Berliner A.; Amir D.; Marciano D.; Elias S.; Katalan S.; Ashkenazi N.; Madmon M.; Gershonov E.; Saphier S. J. Med. Chem. 2019, 62, 5628. |
| [5] | Fujita M.; Obayashi M.; Hiyama T. Tetrahedron 1998, 44, 4135. |
| [6] | Shan C.-C.; Dai K.-Y.; Zhao M.; Xu Y.-H. Eur. J. Org. Chem. 2021, 4054. |
| [7] | Gao P.; Wang G.-Q.; Xi L.-L.; Wang M.-Y.; Li S.-H.; Shi Z.-Z. Chin. J. Chem. 2019, 37, 1009. |
| [8] | Coates G.; Tan H.-Y.; Kalff C.; White A. J. P.; Crimmin M. R. Angew. Chem., Int. Ed. 2019, 58, 12514. |
| [9] | Paioti P.; Mikus M. S.; Lee J.; Koh M. J.; Romiti F.; Torker S.; Pozo J. D.; Hoveyda A. H. J. Am. Chem. Soc. 2019, 141, 19917. |
| [10] | Gao P.; Gao L.-Z.; Xi L.-L.; Zhang Z.-D.; Li S.-H.; Shi Z.-Z. Org. Chem. Front. 2020, 7, 2618. |
| [11] | Yang X.-N.; Guo H.-Y.; Zhou R. Chin. J. Org. Chem. 2023, 43, 2720 (in Chinese). |
| [11] | (杨晓娜, 郭宏宇, 周荣, 有机化学, 2023, 43, 2720.) |
| [12] | Yang X.-H.; Gao H.-W.; Yan J.-L.; Shi L. Chin. J. Org. Chem. 2022, 42, 4122 (in Chinese). |
| [12] | (杨惜晖, 高皓炜, 闫甲乐, 史雷, 有机化学, 2022, 42, 4122.) |
| [13] | Ren L.-Q.; Li N.; Ke J.; He C. Org. Chem. Front. 2022, 9, 6400. |
| [14] | Ghosh S.; Lai D.; Hajra A. Org. Biomol. Chem. 2021, 19, 2399. |
| [15] | Yue F.-Y.; Liu J.-H.; Ma H.-N.; Liu Y.-X.; Dong J.-Y.; Wang Q.-M. Org. Lett. 2022, 24, 4019. |
| [16] | Luo C.; Zhou Y.; Chen H.; Wang T.; Zhang Z.-B.; Han P.; Jing L.-H. Org. Lett. 2022, 24, 4286. |
| [17] | Xu W.-G.; Xia C.-J.; Shao Q.; Zhang Q.; Liu M.-R.; Zhan H.-W.; Wu M.-B. Org. Chem. Front. 2022, 9, 4949. |
| [18] | Takemura N.; Sumida Y.; Ohmiya H. ACS Catal. 2022, 12, 7804. |
| [19] | Liu S.-H.; Pan P.; Fan H.-Q.; Li P.; Wang W.; Zhang Y.-Q. Chem. Sci. 2019, 10, 3817. |
| [20] | Wan Y.; Zhu J.-J.; Yuan Q.-Y.; Liu S.-H.; Zhao J.-H.; Zhang Y.-Q. Org. Lett. 2021, 23, 1406. |
| [21] | Pan P.; Yuan Q.-Y.; Liu S. H.; Zhao J. H.; Zhang Y.-Q. Chin. J. Org. Chem. 2022, 42, 1136 (in Chinese). |
| [21] | (潘鹏, 袁启洋, 刘石惠, 赵建宏, 张永强, 有机化学, 2022, 42, 1136.) |
| [22] | Zhao Y.-M.; Wan Y.; Yuan Q.-Y.; Wei J.-L.; Zhang Y.-Q. Org. Lett. 2023, 25, 1386. |
| [23] | Wan Y.; Zhao Y.-M.; Zhu J.-J.; Yuan Q.-Y.; Wang W.; Zhang Y.-Q. Green Chem. 2023, 25, 256. |
| [24] | Lima F.; Sharma U. K.; Grunenberg L.; Saha D.; Johannsen S.; Sedelmeier J.; Eycken E. V.; Ley S. V. Angew. Chem., Int. Ed. 2017, 56, 15136. |
| [25] | Zhong M.-B.; Pannecoucke X.; Jubault P.; Poisson T. Chem.-Eur. J. 2021, 27, 11818. |
| [26] | Choi G. J.; Zhu Q.-L.; Miller D. C.; Gu C.-J.; Robert R. Nature 2016, 539, 268. |
| [27] | Luo J.; Zhang J. ACS Catal. 2016, 6, 873. |
| [28] | Jeffrey J. L.; Terrett J. A.; MacMillan D. W. C. Science 2015, 349, 1532. |
| [29] | Lang S. B.; Wiles R. J.; Kelly C. B.; Molander G. A. Angew. Chem., Int. Ed. 2017, 56, 15073. |
| [30] | Hu Q.-P.; Cheng J.; Wang Y.; Shi J.; Wang B.-Q.; Hu P.; Zhao K.-Q.; Pan F. Org. Lett. 2021, 23, 4457. |
| [31] | Aelterman M.; Biremond T.; Jubault P.; Poisson T. Chem.-Eur. J. 2022, 28, e202202194. |
| [32] | Shishido R.; Uesugi M.; Takahashi R.; Mita T.; Ishiyama T.; Kubota K.; Ito H. J. Am. Chem. Soc. 2020, 142, 14125. |
| [33] | Carminati D. M.; Decaens J.; Bonnaire S.; Jubaul P.; Fasan R. Angew. Chem., Int. Ed. 2021, 60, 7072. |
| [34] | Fujita T.; Morioka R.; Arita T.; Ichikawa J. Chem. Commun. 2018, 54, 12938. |
/
| 〈 |
|
〉 |