

N-碘代丁二酰亚胺促进烯基肟的串联氧叠氮化反应: 合成叠氮化异噁唑啉类化合物
收稿日期: 2023-04-25
修回日期: 2023-07-17
网络出版日期: 2023-08-15
基金资助
国家自然科学基金(21702043); 河北省自然科学基金(B2021201035)
N-Iodosuccinimide-Promoted Cascade Oxoazidation of Alkenyl Oximes: Synthesis of Azido Isoxazolines
Received date: 2023-04-25
Revised date: 2023-07-17
Online published: 2023-08-15
Supported by
National Natural Science Foundation of China(21702043); Hebei Province Natural Science Foundation(B2021201035)
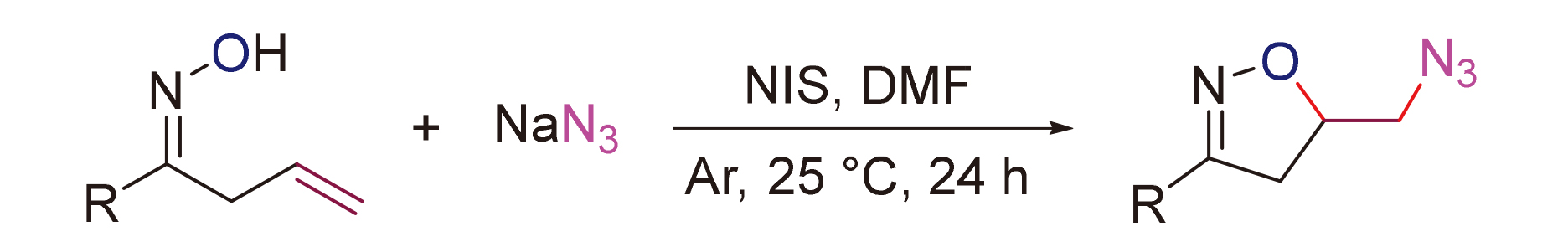
发展了一种N-碘代丁二酰亚胺(NIS)促进下烯基肟和叠氮化钠的氧叠氮化方法, 以良好的收率合成了一系列叠氮化异噁唑啉衍生物. 该反应操作简便且条件温和. 机理研究表明, 该反应可能经历了一个亲电碘环化/亲核取代的串联历程.
关键词: N-碘代丁二酰亚胺(NIS); 烯基肟; 氧叠氮化; 异噁唑啉
刘浩然 , 俞骏豪 , 曹同阳 , 齐林 , 王力竞 . N-碘代丁二酰亚胺促进烯基肟的串联氧叠氮化反应: 合成叠氮化异噁唑啉类化合物[J]. 有机化学, 2023 , 43(12) : 4220 -4226 . DOI: 10.6023/cjoc202304033
A N-iodosuccinimide (NIS)-promoted cascade oxoazidation of alkenyl oximes with NaN3 was developed, and a series of azido isoxazoline derivatives were synthesized in good yields. The reactions are easy to be conducted under mild conditions. The mechanism study shows that the reaction might involve a cascade electrophilic iodocyclization/nucleophilic substitution pathway.

Key words: N-iodosuccinimide (NIS); alkenyl oximes; oxoazidation; isoxazolines
| [1] | (a) McDonald R. I.; Liu G.; Stahl S. S. Chem. Rev. 2011, 111, 2981. |
| [1] | (b) Patel M.; Desai B.; Sheth A.; Dholakiya B. Z.; Naveen T. Asian J. Org. Chem. 2021, 10, 3201. |
| [1] | (c) Li M.; Zhao D.; Sun K. Chin. J. Org. Chem. 2022, 42, 4152. (in Chinese) |
| [1] | (李猛, 赵东阳, 孙凯, 有机化学, 2022, 42, 4152.) |
| [2] | (a) Sivaguru P.; Ning Y.; Bi X. Chem. Rev. 2021, 121, 4253. |
| [2] | (b) Wu K.; Liang Y.; Jiao N. Molecules 2016, 21, 352. |
| [3] | (a) Foschi F.; Loro C.; Sala R.; Oble J.; Lo Presti L.; Beccalli E. M.; Poli G.; Broggini G. Org. Lett. 2020, 22, 1402. |
| [3] | (b) Guo J.; Chen S.; Liu J.; Guo J.; Chen W.; Cai Q.; Liu P.; Sun P. Eur. J. Org. Chem. 2017, 4773. |
| [3] | (c) Li X.; Qi X.; Hou C.; Chen P.; Liu G. Angew. Chem., Int. Ed. 2020, 59, 17239. |
| [3] | (d) Ortiz G. X., Jr.; Kang B.; Wang Q. J. Org. Chem. 2014, 79, 571. |
| [3] | (e) Sequeira F. C.; Turnpenny B. W.; Chemler S. R. Angew. Chem., Int. Ed. 2010, 49, 6365. |
| [3] | (f) Shen K.; Wang Q. J. Am. Chem. Soc. 2017, 139, 13110. |
| [3] | (g) Wang L. J.; Ren P. X.; Qi L.; Chen M.; Lu Y. L.; Zhao J. Y.; Liu R.; Chen J. M.; Li W. Org. Lett. 2018, 20, 4411. |
| [3] | (h) Zhang P.; Sun W.; Li G.; Hong L.; Wang R. Chem. Commun. 2015, 51, 12293. |
| [4] | Sequeira F. C.; Chemler S. R. Org. Lett. 2012, 14, 4482. |
| [5] | Zhu L.; Yu H.; Xu Z.; Jiang X.; Lin L.; Wang R. Org. Lett. 2014, 16, 1562. |
| [6] | Yin H.; Wang T.; Jiao N. Org. Lett. 2014, 16, 2302. |
| [7] | Zhu R.; Buchwald S. L. J. Am. Chem. Soc. 2015, 137, 8069. |
| [8] | Cao T. Y.; Qi L.; Dong W.; Yan Z. M.; Ji S. C.; Du J. L.; Zhang L.; Li W.; Wang L. J. J. Org. Chem. 2022, 87, 16578. |
| [9] | Yang S.; Li H.; Li P.; Yang J.; Wang L. Org. Biomol. Chem. 2020, 18, 715. |
| [10] | Yu W.; Wang P.-L.; Xu K.; Li H. Asian J. Org. Chem. 2021, 10, 831. |
| [11] | Wang L.J.; Chen M.; Qi L.; Xu Z.; Li W. Chem. Commun. 2017, 53, 2056. |
| [12] | Wu X.-B.; Gao Q.; Fan J.-J.; Zhao Z.-Y.; Tu X.-Q.; Cao H.-Q.; Yu J. Org. Lett. 2021, 23, 9134. |
/
| 〈 |
|
〉 |