

芳基乙烯基硅烷与芳基卤代物的Hiyama偶联反应
收稿日期: 2023-06-28
修回日期: 2023-07-23
网络出版日期: 2023-08-15
基金资助
国家自然科学基金(22071084); 国家自然科学基金(22271127); 中央高校基本科研业务费专项资金(lzujbky-2022-ey01)
Hiyama Cross-Coupling Reaction of Aryl Vinylsilanes and Aryl Halides
Received date: 2023-06-28
Revised date: 2023-07-23
Online published: 2023-08-15
Supported by
National Natural Science Foundation of China(22071084); National Natural Science Foundation of China(22271127); Fundamental Research Funds for the Central Universities(lzujbky-2022-ey01)
马伟源 , 戴惠芳 , 亢少林 , 张天麟 , 舒兴中 . 芳基乙烯基硅烷与芳基卤代物的Hiyama偶联反应[J]. 有机化学, 2023 , 43(10) : 3614 -3622 . DOI: 10.6023/cjoc202306025
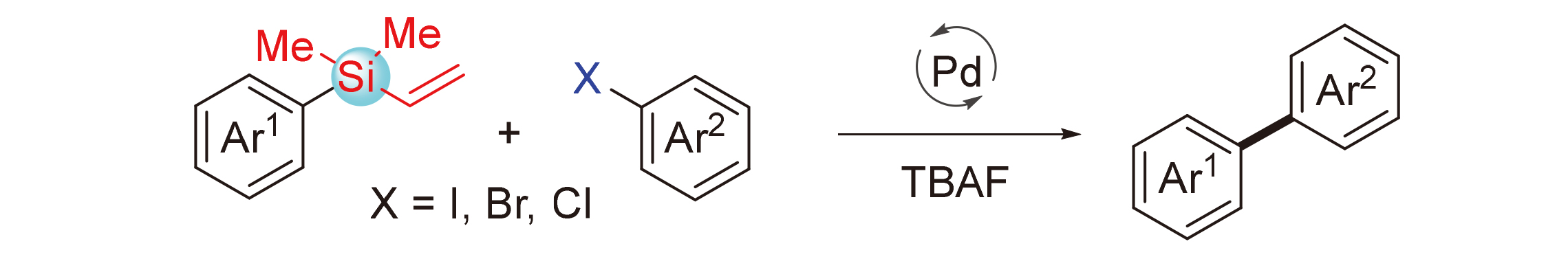
The Hiyama coupling reaction has emerged as a wildly used method for the construction of C—C bonds, especially in the fields of aryl-aryl and aryl-alkenyl coupling reactions. In general, this protocol highly relies on reactive but unstable silicon reagents such as R—SiF3 and R—Si(OMe)3. The development of Hiyama coupling reaction involving stable organosilanes is in high demand. In this manuscript, a palladium-catalyzed cross-coupling reaction of aryl vinylsilanes and aryl halides is reported, leading to the formation of Ar—Ar bonds. The reaction has shown good functional group compatibility and offered convenient access to biaryl compounds.
| [1] | (a) Thomson R. H. The Chemistry of Natural Products, Blackie and Son, Glasgow, UK, 1985. |
| [1] | (b) Brunel J. M. Chem. Rev. 2005, 105, 857. |
| [1] | (c) Corbet J. P.; Mignani G. Chem. Rev. 2006, 106, 2651. |
| [2] | (a) Nakao Y.; Hiyama T. Chem. Soc. Rev. 2011, 40, 4893. |
| [2] | (b) Komiyama T.; Minami Y.; Hiyama T. ACS Catal. 2017, 7, 631. |
| [3] | (a) Chan T. H.; Fleming I. Synthesis 1979, 761. |
| [3] | (b) Denmark S. E.; Ambrosi A. Org. Process Res. Dev. 2015, 19, 982. |
| [4] | (a) Hiyama T.; Oestreich M. Organosilicon Chemistry: Novel Approaches and Reactions, Wiley-VCH, Weinheim, 2019. |
| [4] | (b) Denmark S. E.; Regens C. S. Acc. Chem. Res. 2008, 41, 1486. |
| [4] | (c) Sore H. F.; Galloway W. R. J. D.; Spring D. R. Chem. Soc. Rev. 2012, 41, 1845. |
| [4] | (d) Foubelo F.; Nájera C.; Yus M. Chem. Rec. 2016, 16, 2521. |
| [5] | (a) Minami Y.; Hiyama T. Chem.-Eur. J. 2019, 25, 391. |
| [5] | (b) Komiyama T.; Minami Y.; Hiyama T. Synlett 2017, 28,1873 |
| [6] | (a) Duan J.; Wang K.; Xu G.-L.; Kang S.; Qi L.; Liu X.-Y.; Shu X.-Z. Angew. Chem., Int. Ed. 2020, 59, 23083. |
| [6] | (b) Duan J.; Wang Y.; Qi L.; Guo P.; Pang X.; Shu X.-Z. Org. Lett. 2021, 23, 7855. |
| [7] | (a) Itami K.; Nokami T.; Yoshida J.-I. J. Am. Chem. Soc. 2001, 123, 5600. |
| [7] | (b) Bergueiro J.; Montenegro J.; Cambeiro F.; Saá C.; López S. Chem.-Eur. J. 2012, 18, 4401. |
| [7] | (c) Hosoi K.; Nozaki K.; Hiyama T. Chem. Lett. 2002, 31, 138. |
| [7] | (d) Vitale M.; Prestat G.; Lopes D.; Madec D.; Kammerer C.; Poli G.; Girnita L. J. Org. Chem. 2008, 73, 5795. |
| [7] | (e) Lorion M. M.; Matt B.; Alves S.; Proust A.; Poli G.; Oble J.; Izzet G. Chem.-Eur. J. 2013, 19, 12607. |
| [7] | (f) Katayama H.; Nagao M.; Ozawa F.; Ikegami M.; Arai T. J. Org. Chem. 2006, 71, 2699. |
| [7] | (g) Anderson J. C.; Munday R. H. J. Org. Chem. 2004, 69, 8971. |
| [7] | (h) Pawley S. B.; Conner A. M.; Omer H. M.; Watson D. A. ACS Catal. 2022, 12, 13108. |
| [8] | (a) Grushin V. V.; Alper H. Chem. Rev. 1994, 94, 1047. |
| [8] | (b) Littke A. F.; Fu G. C. Angew. Chem., Int. Ed. 2002, 41, 4176. |
| [8] | (c) Yuen O. Y.; So C. M.; Man H. W.; Kwong F. Y. Chem.-Eur. J. 2016, 22, 6471. |
| [9] | Pierrat P.; Gros P.; Fort Y. Org. Lett. 2005, 7, 697. |
| [10] | (a) Lühning L. H.; Rosien M.; Doye S. Synlett 2017, 28, 2489. |
| [10] | (b) Kratz T.; Steinbach P.; Breitenlechner S.; Storch G.; Bann- warth C.; Bach T. J. Am. Chem. Soc. 2022, 144, 10133. |
| [11] | Morioka T.; Nishizawa A.; Furukawa T.; Tobisu M.; Chatani N. J. Am. Chem. Soc. 2017, 139, 1416. |
| [12] | Cao Z.-C.; Luo Q.-Y.; Shi Z.-J. Org. Lett. 2016, 18, 5978. |
| [13] | Guan B.-T.; Wang Y.; Li B.-J.; Yu D.-G.; Shi Z.-J. J. Am. Chem. Soc. 2008, 130, 14468. |
| [14] | Chen H.; Huang Z.; Hu X.; Tang G.; Xu P.; Zhao Y.; Cheng C.-H. J. Org. Chem. 2011, 76, 2338. |
| [15] | Hua X.; Masson-Makdissi J.; Sullivan R. J.; Newman S. G. Org. Lett. 2016, 18, 5312. |
| [16] | Qin C.; Lu W. J. Org. Chem. 2008, 73, 7424. |
| [17] | Zhao C.-W.; Ma J.-P.; Liu Q.-K.; Yu Y.; Wang P.; Li Y.-A.; Wang K.; Dong Y.-B. Green Chem. 2013, 15, 3150. |
| [18] | Vila C.; Cembellín S.; Hornillos V.; Giannerini M.; Fa?anás- Mastral M.; Feringa B. L. Eur. J. Org. Chem. 2015, 21, 15520. |
| [19] | Zhu S.; Xiao Y.; Guo Z.; Jiang H. Org. Lett. 2013, 15, 898. |
| [20] | Minami H.; Wang X.; Wang C.; Uchiyama M. Eur. J. Org. Chem. 2013, 2013, 7891. |
| [21] | Mohamed R. K.; Mondal S.; Gold B.; Evoniuk C. J.; Banerjee T.; Hanson K.; Alabugin I. V. J. Am. Chem. Soc. 2015, 137, 6335. |
| [22] | Baxendale I. R.; Griffiths-Jones C. M.; Ley S. V.; Tranmer G. K. Chem.-Eur. J. 2006, 12, 4407. |
| [23] | Liang Z.; Ju L.; Xie Y.; Huang L.; Zhang Y. Chem.-Eur. J. 2012, 18, 15816. |
| [24] | Reddy V. P.; Qiu R.; Iwasaki T.; Kambe N. Org. Lett. 2013, 15, 1290. |
| [25] | Ye M.; Gao G.-L.; Edmunds A. J. F.; Worthington P. A.; Morris J. A.; Yu J.-Q. J. Am. Chem. Soc. 2011, 133, 19090. |
| [26] | Kaur M.; U Din Reshi N.; Patra K.; Bhattacherya A.; Kunnikuruvan S.; Bera J. K. Chem.-Eur. J. 2021, 27, 10737. |
| [27] | Budén M. E.; Guastavino J. F.; Rossi R. A. Org. Lett. 2013, 15, 1174. |
| [28] | Pan C.; Zhu J.; Chen R.; Yu J.-T. Org. Biomol. Chem. 2017, 15, 6467. |
| [29] | Pavia C.; Ballerini E.; Bivona L. A.; Giacalone F.; Aprile C.; Vaccaro L.; Gruttadauria M. Adv. Synth. Catal. 2013, 355, 2007. |
| [30] | Gu P.; Xu Q.; Shi M. Synlett 2013, 24, 1255. |
| [31] | Mowery M. E.; DeShong P. J. Org. Chem. 1999, 64, 3266. |
| [32] | Salanouve E.; Bouzemame G.; Blanchard S.; Derat E.; Murr M. D.-E.; Fensterbank L. Chem.-Eur. J. 2014, 20, 4754. |
| [33] | Liang Q.; Xing P.; Huang Z.; Dong J.; Sharpless K. B.; Li X.; Jiang B. Org. Lett. 2015, 17, 1942. |
| [34] | Witzel S.; Xie J.; Rudolph M.; Hashmi A. S. K. Adv. Synth. Catal. 2017, 359, 1522. |
| [35] | Wang D.-Y.; Wang C.; Uchiyama M. J. Am. Chem. Soc. 2015, 137, 10488. |
| [36] | Shrestha B.; Thapa S.; Gurung S. K.; Pike R. A. S.; Giri R. J. Org. Chem. 2016, 81, 787. |
/
| 〈 |
|
〉 |