有机化学 ›› 2023, Vol. 43 ›› Issue (10): 3614-3622.DOI: 10.6023/cjoc202306025 上一篇 下一篇
所属专题: 有机硅化学专辑-2023
研究论文
马伟源a, 戴惠芳b, 亢少林a, 张天麟a, 舒兴中a,*( )
)
收稿日期:2023-06-28
修回日期:2023-07-23
发布日期:2023-08-16
作者简介:基金资助:
Wei-Yuan Maa, Huifang Daib, Shaolin Kanga, Tianlin Zhanga, Xing-Zhong Shua( )
)
Received:2023-06-28
Revised:2023-07-23
Published:2023-08-16
Contact:
*E-mail: About author:Supported by:文章分享
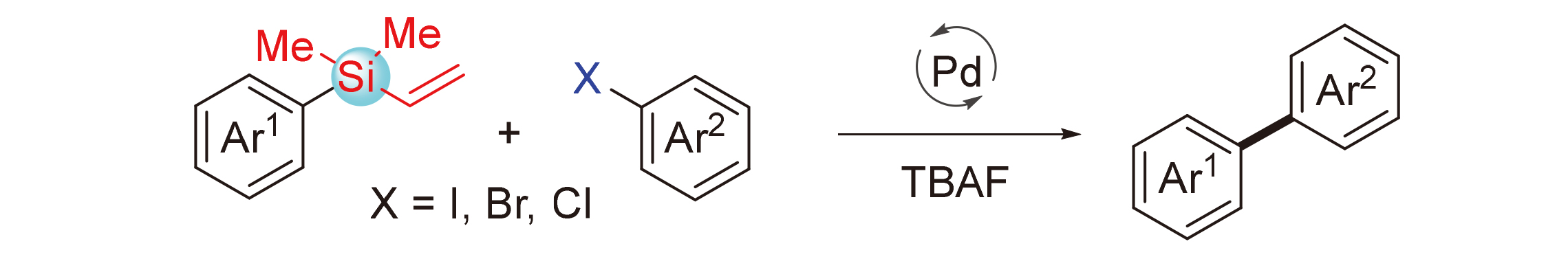
Hiyama偶联反应已经发展成为一种构筑C—C键的常用方法, 尤其是在芳基-芳基和芳基-烯基偶联反应领域. Hiyama偶联反应通常需要使用R—SiF3、R—Si(OMe)3等活性高但稳定性差的有机硅试剂, 发展基于稳定硅烷的Hiyama偶联反应是该领域重要的研究方向. 报道了一类钯催化芳基乙烯基硅烷和芳基卤代物的交叉偶联反应, 利用芳基乙烯基硅烷实现芳基化反应. 反应具有较好的官能团兼容性, 为制备二芳基类化合物提供了一种简便高效的途径.
马伟源, 戴惠芳, 亢少林, 张天麟, 舒兴中. 芳基乙烯基硅烷与芳基卤代物的Hiyama偶联反应[J]. 有机化学, 2023, 43(10): 3614-3622.
Wei-Yuan Ma, Huifang Dai, Shaolin Kang, Tianlin Zhang, Xing-Zhong Shu. Hiyama Cross-Coupling Reaction of Aryl Vinylsilanes and Aryl Halides[J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3614-3622.
| Entry | Change of conditions | Yield/% of 3a |
|---|---|---|
| 1 | None | 89 (85)c |
| 2 | Pd(PPh3)2Cl2b | 60 |
| 3 | Pd(dba)2b | 35 |
| 4 | PdCl2b | 15 |
| 5 | CuCl | 28 |
| 6 | CuF2 | 22 |
| 7 | CsF | 51 |
| 8 | KF | 19 |
| 9 | No Pd, F, or Cu | 0 |
| 10 | PhBr was usedd | 88c |
| Entry | Change of conditions | Yield/% of 3a |
|---|---|---|
| 1 | None | 89 (85)c |
| 2 | Pd(PPh3)2Cl2b | 60 |
| 3 | Pd(dba)2b | 35 |
| 4 | PdCl2b | 15 |
| 5 | CuCl | 28 |
| 6 | CuF2 | 22 |
| 7 | CsF | 51 |
| 8 | KF | 19 |
| 9 | No Pd, F, or Cu | 0 |
| 10 | PhBr was usedd | 88c |
| [1] |
(a) Thomson R. H. The Chemistry of Natural Products, Blackie and Son, Glasgow, UK, 1985.
|
|
(b) Brunel J. M. Chem. Rev. 2005, 105, 857.
doi: 10.1021/cr040079g |
|
|
(c) Corbet J. P.; Mignani G. Chem. Rev. 2006, 106, 2651.
doi: 10.1021/cr0505268 |
|
| [2] |
(a) Nakao Y.; Hiyama T. Chem. Soc. Rev. 2011, 40, 4893.
doi: 10.1039/c1cs15122c |
|
(b) Komiyama T.; Minami Y.; Hiyama T. ACS Catal. 2017, 7, 631.
doi: 10.1021/acscatal.6b02374 |
|
| [3] |
(a) Chan T. H.; Fleming I. Synthesis 1979, 761.
pmid: 26478695 |
|
(b) Denmark S. E.; Ambrosi A. Org. Process Res. Dev. 2015, 19, 982.
pmid: 26478695 |
|
| [4] |
(a) Hiyama T.; Oestreich M. Organosilicon Chemistry: Novel Approaches and Reactions, Wiley-VCH, Weinheim, 2019.
|
|
(b) Denmark S. E.; Regens C. S. Acc. Chem. Res. 2008, 41, 1486.
doi: 10.1021/ar800037p |
|
|
(c) Sore H. F.; Galloway W. R. J. D.; Spring D. R. Chem. Soc. Rev. 2012, 41, 1845.
doi: 10.1039/C1CS15181A |
|
|
(d) Foubelo F.; Nájera C.; Yus M. Chem. Rec. 2016, 16, 2521.
doi: 10.1002/tcr.v16.6 |
|
| [5] |
(a) Minami Y.; Hiyama T. Chem.-Eur. J. 2019, 25, 391.
doi: 10.1002/chem.v25.2 |
|
(b) Komiyama T.; Minami Y.; Hiyama T. Synlett 2017, 28,1873
|
|
| [6] |
(a) Duan J.; Wang K.; Xu G.-L.; Kang S.; Qi L.; Liu X.-Y.; Shu X.-Z. Angew. Chem., Int. Ed. 2020, 59, 23083.
doi: 10.1002/anie.v59.51 |
|
(b) Duan J.; Wang Y.; Qi L.; Guo P.; Pang X.; Shu X.-Z. Org. Lett. 2021, 23, 7855.
doi: 10.1021/acs.orglett.1c02874 |
|
| [7] |
(a) Itami K.; Nokami T.; Yoshida J.-I. J. Am. Chem. Soc. 2001, 123, 5600.
pmid: 36817085 |
|
(b) Bergueiro J.; Montenegro J.; Cambeiro F.; Saá C.; López S. Chem.-Eur. J. 2012, 18, 4401.
doi: 10.1002/chem.201103360 pmid: 36817085 |
|
|
(c) Hosoi K.; Nozaki K.; Hiyama T. Chem. Lett. 2002, 31, 138.
doi: 10.1246/cl.2002.138 pmid: 36817085 |
|
|
(d) Vitale M.; Prestat G.; Lopes D.; Madec D.; Kammerer C.; Poli G.; Girnita L. J. Org. Chem. 2008, 73, 5795.
doi: 10.1021/jo800707q pmid: 36817085 |
|
|
(e) Lorion M. M.; Matt B.; Alves S.; Proust A.; Poli G.; Oble J.; Izzet G. Chem.-Eur. J. 2013, 19, 12607.
doi: 10.1002/chem.v19.38 pmid: 36817085 |
|
|
(f) Katayama H.; Nagao M.; Ozawa F.; Ikegami M.; Arai T. J. Org. Chem. 2006, 71, 2699.
doi: 10.1021/jo052602c pmid: 36817085 |
|
|
(g) Anderson J. C.; Munday R. H. J. Org. Chem. 2004, 69, 8971.
pmid: 36817085 |
|
|
(h) Pawley S. B.; Conner A. M.; Omer H. M.; Watson D. A. ACS Catal. 2022, 12, 13108.
doi: 10.1021/acscatal.2c03981 pmid: 36817085 |
|
| [8] |
(a) Grushin V. V.; Alper H. Chem. Rev. 1994, 94, 1047.
doi: 10.1021/cr00028a008 |
|
(b) Littke A. F.; Fu G. C. Angew. Chem., Int. Ed. 2002, 41, 4176.
|
|
|
(c) Yuen O. Y.; So C. M.; Man H. W.; Kwong F. Y. Chem.-Eur. J. 2016, 22, 6471.
doi: 10.1002/chem.v22.19 |
|
| [9] |
Pierrat P.; Gros P.; Fort Y. Org. Lett. 2005, 7, 697.
doi: 10.1021/ol047482u |
| [10] |
(a) Lühning L. H.; Rosien M.; Doye S. Synlett 2017, 28, 2489.
doi: 10.1055/s-0036-1589048 |
|
(b) Kratz T.; Steinbach P.; Breitenlechner S.; Storch G.; Bann- warth C.; Bach T. J. Am. Chem. Soc. 2022, 144, 10133.
doi: 10.1021/jacs.2c02511 |
|
| [11] |
Morioka T.; Nishizawa A.; Furukawa T.; Tobisu M.; Chatani N. J. Am. Chem. Soc. 2017, 139, 1416.
doi: 10.1021/jacs.6b12293 |
| [12] |
Cao Z.-C.; Luo Q.-Y.; Shi Z.-J. Org. Lett. 2016, 18, 5978.
doi: 10.1021/acs.orglett.6b02656 |
| [13] |
Guan B.-T.; Wang Y.; Li B.-J.; Yu D.-G.; Shi Z.-J. J. Am. Chem. Soc. 2008, 130, 14468.
doi: 10.1021/ja8056503 |
| [14] |
Chen H.; Huang Z.; Hu X.; Tang G.; Xu P.; Zhao Y.; Cheng C.-H. J. Org. Chem. 2011, 76, 2338.
doi: 10.1021/jo2000034 pmid: 21388215 |
| [15] |
Hua X.; Masson-Makdissi J.; Sullivan R. J.; Newman S. G. Org. Lett. 2016, 18, 5312.
doi: 10.1021/acs.orglett.6b02631 |
| [16] |
Qin C.; Lu W. J. Org. Chem. 2008, 73, 7424.
doi: 10.1021/jo801345b |
| [17] |
Zhao C.-W.; Ma J.-P.; Liu Q.-K.; Yu Y.; Wang P.; Li Y.-A.; Wang K.; Dong Y.-B. Green Chem. 2013, 15, 3150.
doi: 10.1039/c3gc41154k |
| [18] |
Vila C.; Cembellín S.; Hornillos V.; Giannerini M.; Fañanás- Mastral M.; Feringa B. L. Eur. J. Org. Chem. 2015, 21, 15520.
|
| [19] |
Zhu S.; Xiao Y.; Guo Z.; Jiang H. Org. Lett. 2013, 15, 898.
doi: 10.1021/ol4000394 |
| [20] |
Minami H.; Wang X.; Wang C.; Uchiyama M. Eur. J. Org. Chem. 2013, 2013, 7891.
doi: 10.1002/ejoc.v2013.35 |
| [21] |
Mohamed R. K.; Mondal S.; Gold B.; Evoniuk C. J.; Banerjee T.; Hanson K.; Alabugin I. V. J. Am. Chem. Soc. 2015, 137, 6335.
doi: 10.1021/jacs.5b02373 pmid: 25906261 |
| [22] |
Baxendale I. R.; Griffiths-Jones C. M.; Ley S. V.; Tranmer G. K. Chem.-Eur. J. 2006, 12, 4407.
pmid: 16586523 |
| [23] |
Liang Z.; Ju L.; Xie Y.; Huang L.; Zhang Y. Chem.-Eur. J. 2012, 18, 15816.
doi: 10.1002/chem.v18.49 |
| [24] |
Reddy V. P.; Qiu R.; Iwasaki T.; Kambe N. Org. Lett. 2013, 15, 1290.
doi: 10.1021/ol400230y |
| [25] |
Ye M.; Gao G.-L.; Edmunds A. J. F.; Worthington P. A.; Morris J. A.; Yu J.-Q. J. Am. Chem. Soc. 2011, 133, 19090.
doi: 10.1021/ja209510q |
| [26] |
Kaur M.; U Din Reshi N.; Patra K.; Bhattacherya A.; Kunnikuruvan S.; Bera J. K. Chem.-Eur. J. 2021, 27, 10737.
doi: 10.1002/chem.v27.41 |
| [27] |
Budén M. E.; Guastavino J. F.; Rossi R. A. Org. Lett. 2013, 15, 1174.
doi: 10.1021/ol3034687 |
| [28] |
Pan C.; Zhu J.; Chen R.; Yu J.-T. Org. Biomol. Chem. 2017, 15, 6467.
doi: 10.1039/C7OB01564J |
| [29] |
Pavia C.; Ballerini E.; Bivona L. A.; Giacalone F.; Aprile C.; Vaccaro L.; Gruttadauria M. Adv. Synth. Catal. 2013, 355, 2007.
doi: 10.1002/adsc.v355.10 |
| [30] |
Gu P.; Xu Q.; Shi M. Synlett 2013, 24, 1255.
doi: 10.1055/s-00000083 |
| [31] |
Mowery M. E.; DeShong P. J. Org. Chem. 1999, 64, 3266.
pmid: 11674429 |
| [32] |
Salanouve E.; Bouzemame G.; Blanchard S.; Derat E.; Murr M. D.-E.; Fensterbank L. Chem.-Eur. J. 2014, 20, 4754.
doi: 10.1002/chem.201304459 pmid: 24634349 |
| [33] |
Liang Q.; Xing P.; Huang Z.; Dong J.; Sharpless K. B.; Li X.; Jiang B. Org. Lett. 2015, 17, 1942.
doi: 10.1021/acs.orglett.5b00654 |
| [34] |
Witzel S.; Xie J.; Rudolph M.; Hashmi A. S. K. Adv. Synth. Catal. 2017, 359, 1522.
doi: 10.1002/adsc.v359.9 |
| [35] |
Wang D.-Y.; Wang C.; Uchiyama M. J. Am. Chem. Soc. 2015, 137, 10488.
doi: 10.1021/jacs.5b06587 |
| [36] |
Shrestha B.; Thapa S.; Gurung S. K.; Pike R. A. S.; Giri R. J. Org. Chem. 2016, 81, 787.
doi: 10.1021/acs.joc.5b02077 |
| [1] | 陈雯雯, 张琴, 张松月, 黄芳芳, 张馨尹, 贾建峰. 无光催化剂条件下可见光诱导炔基碘和亚磺酸钠偶联反应[J]. 有机化学, 2024, 44(2): 584-592. |
| [2] | 陈祖佳, 宇世伟, 周永军, 李焕清, 邱琪雯, 李妙欣, 汪朝阳. BF3•OEt2作为催化剂与合成子在有机合成中的应用进展[J]. 有机化学, 2023, 43(9): 3107-3118. |
| [3] | 芦军, 李奇闯, 梁仁校, 贾义霞. 镍催化吡啶/喹啉鎓盐分子内去芳构化芳基加成反应[J]. 有机化学, 2023, 43(5): 1875-1882. |
| [4] | 刘宁, 爨晓丹, 李慧, 段希焱. 烯胺酮α-官能团化反应的研究进展[J]. 有机化学, 2023, 43(2): 602-621. |
| [5] | 陈东平, 杨春红, 李明, 赵国孝, 王文鹏, 王喜存, 权正军. 芳炔参与的三组分芳基化反应进展[J]. 有机化学, 2023, 43(2): 503-525. |
| [6] | 马彪, 章淼淼, 李占宇, 彭进松, 陈春霞. 无过渡金属催化的Suzuki-Type交叉偶联反应研究进展[J]. 有机化学, 2023, 43(2): 455-470. |
| [7] | 刘婷婷, 胡宇才, 沈安. 亚胺配体协同氮杂环卡宾钯配合物催化碳碳偶联反应的作用机制[J]. 有机化学, 2023, 43(2): 622-628. |
| [8] | 秦思凝. 芳香卤代物C—S偶联反应的研究进展[J]. 有机化学, 2023, 43(11): 3761-3783. |
| [9] | 孙美娇, 谭晶, 谭玉, 彭进松, 陈春霞. 钯催化3-(2-氨基嘧啶-4-基)吲哚2位C—H键芳基化反应的研究[J]. 有机化学, 2023, 43(11): 3945-3959. |
| [10] | 王静, 吴琳琳, 王倩. 新型茚并芴-6,12-二酮衍生物的合成与性能研究[J]. 有机化学, 2023, 43(1): 223-228. |
| [11] | 贾雪锋, 仝向娟. 铜(II)配合物催化Chan-Lam偶联反应研究进展[J]. 有机化学, 2022, 42(9): 2640-2658. |
| [12] | 宋戈洋, 薛东. 光促进过渡金属催化的C-杂原子键偶联反应进展[J]. 有机化学, 2022, 42(8): 2275-2299. |
| [13] | 白瑞, 刘旭娟, 罗文钰, 刘珊珊, 焦林郁. 多相催化体系下Chan-Lam偶联反应的研究进展[J]. 有机化学, 2022, 42(8): 2342-2354. |
| [14] | 张力之, 廖永剑, 陈宁, 黄磊, 周敏. 叔丁醇钾促进的环化和偶联反应[J]. 有机化学, 2022, 42(7): 1950-1959. |
| [15] | 余卫国, 王灵娜, 俞晓聪, 罗书平. 荧光染料和镍协同催化的脱羧羰基化反应[J]. 有机化学, 2022, 42(4): 1216-1223. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||