

铑(III)催化苯甲亚胺酸乙酯和CF3-亚胺氧锍叶立德C—H 活化/环化反应合成CF3-1H-苯并[de][1,8]萘吡啶
收稿日期: 2023-06-01
修回日期: 2023-07-26
网络出版日期: 2023-08-22
基金资助
国家自然科学基金(22071068); 及华侨大学分析测试中心资助项目.
Rhodium(III)-Catalyzed Synthesis of CF3-1H-benzo[de][1,8]naph-thyridines via C—H Activation/Annulation of Benzimidates and CF3-Imidoyl Sulfoxonium Ylides
Received date: 2023-06-01
Revised date: 2023-07-26
Online published: 2023-08-22
Supported by
National Natural Science Foundation of China(22071068); Instrumental Analysis Center of Huaqiao University.
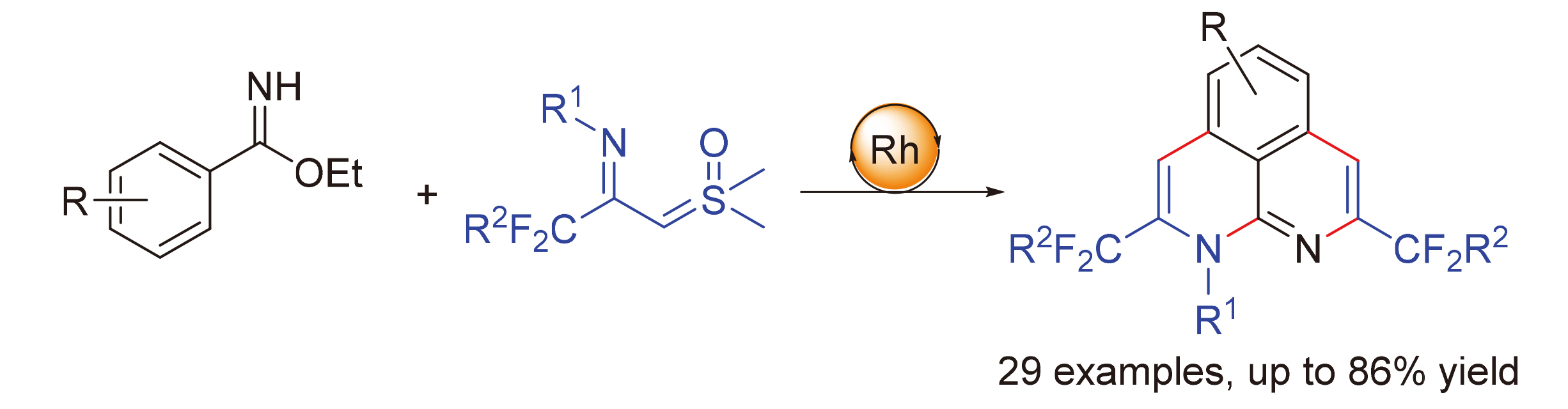
在铑催化下, 实现了苯甲亚胺酸乙酯类化合物与CF3-亚胺叶立德的C—H活化/环化多米诺反应. 该合成策略具有良好的官能团耐受性和底物的普适性, 合成了一系列含三氟甲基的1H-苯并[de][1,8]萘吡啶, 产率为22%~86%.
关键词: 铑催化; 苯甲胺酸乙酯; 三氟甲基; 氧锍叶立德; 苯并[de][1,8]萘吡啶
文思 , 丁宇浩 , 田青于 , 葛进 , 程国林 . 铑(III)催化苯甲亚胺酸乙酯和CF3-亚胺氧锍叶立德C—H 活化/环化反应合成CF3-1H-苯并[de][1,8]萘吡啶[J]. 有机化学, 2024 , 44(1) : 291 -300 . DOI: 10.6023/cjoc202306002
A rhodium-catalyzed C—H activation/annulation domino reaction of benzimidates and CF3-imidoyl sulfoxonium ylides has been achieved. This synthetic strategy has good functional groups tolerance and universality of substrates, and a series of trifluoromethyl-containing 1H-benzo[de][1,8]naphthyridines were synthesized in 22%~86% yields.

| [1] | (a) Zhuang, L.; Wai, J. S.; Embrey, M. W.; Fisher, T. E.; Egbertson, M. S.; Payne, L. S.; Guare, J. P.; Vacca, J. P.; Hazuda, D. J.; Felock, P. J.; Wolfe, A. L.; Stillmock, K. A.; Witmer, M. V.; Moyer, G.; Schleif, W. A.; Gabryelski, L. J.; Leonard, Y. M.; Lynch, J. J.; Michelson, S. R.; Young, S. D. J. Med. Chem. 2003, 46, 453. |
| [1] | (b) Embrey, M. W.; Wai, J. S.; Funk, T. W.; Homnick, C. F.; Perlow, D. S.; Young, S. D.; Vacca, J. P.; Hazuda, D. J.; Felock, P. J.; Stillmock, K. A.; Witmer, M. V.; Moyer, G.; Schleif, W. A.; Gabryelski, L. J.; Jin, L.; Chen, I.-W.; Ellis, J. D.; Wong, B. K.; Lin, J. H.; Leonard, Y. M.; Tsou, N. N.; Zhuang, L. Bioorg. Med. Chem. Lett. 2005, 15, 4550. |
| [2] | (a) Calcul, L.; Longeon, A.; Mourabit, A. A.; Guyot, M.; Bourguet-Kondracki, M.-L. Tetrahedron 2003, 59, 6539. |
| [2] | (b) Aoki, S.; Kong, D.; Suna, H.; Sowa, Y.; Sakai, T.; Setiawan, A.; Kobayashi, M. Biochem. Biophys. Res. Commun. 2006, 342, 101. |
| [3] | (a) Stockner, F.; Beckert, R.; Gleich, D.; Birckner, E.; Gunther, W.; Gorls, H.; Vaughan, G. Eur. J. Org. Chem. 2007, 2007, 1237. |
| [3] | (b) Matschke, M.; Beckert, R.; Würthwein, E.-U.; Gorls, H. Synlett 2008, 2633. |
| [3] | (c) Bunz, U. H. F. Chem.-Eur. J. 2009, 15, 6780. |
| [3] | (d) Tang, Q.; Liu, J.; Chan, H. S.; Miao, Q. Chem.-Eur. J. 2009, 15, 3965. |
| [3] | (e) Behof, W. J.; Wang, D.; Niu, W.; Gorman, C. B. Org. Lett. 2010, 12, 2146. |
| [4] | (a) Chen, X.; Engle, K. M.; Wang, D.-H.; Yu, J.-Q. Angew. Chem., Int. Ed. 2009, 48, 5094. |
| [4] | (b) Colby, D. A.; Bergman, R. G.; Ellman, J. A. Chem. Rev. 2010, 110, 624. |
| [4] | (c) Lyons, T. W.; Sanford, M. S. Chem. Rev. 2010, 110, 1147. |
| [5] | (a) Mochida, S.; Umeda, N.; Hirano, K.; Satoh, T.; Miura, M. Chem. Lett. 2010, 39, 744. |
| [5] | (b) Huang, J.-R.; Dong, L.; Han, B.; Peng, C.; Chen, Y.-C. Chem.-Eur. J. 2012, 18, 8896. |
| [5] | (c) Tan, X.; Liu, B.; Li, X.; Li, B.; Xu, S.; Song, H.; Wang, B. J. Am. Chem. Soc. 2012, 134, 16163. |
| [6] | Jayakumar, J.; Parthasarathy, K.; Chen, Y.-H.; Lee, T.-H.; Chuang, S.-C.; Cheng, C.-H. Angew. Chem., Int. Ed. 2014, 53, 9889. |
| [7] | Huang, T.; Wang, T.; Shi, Y.; Chen, J.; Guo, X.; Guo, X.; Guo, X.; Wu, Z.; Peng, D.; Wang, L.; Li, H.; Hai, L.; Wu, Y. Org. Lett. 2021, 23, 1548. |
| [8] | (a) Barday, M.; Janot, C.; Halcovitch, N. R.; Muir, J.; Aissa, C. Angew. Chem., Int. Ed. 2017, 56, 13117. |
| [8] | (b) Xu, Y.; Xu, Y.; Zheng, G.; Li, X. Org. Lett. 2017, 19, 5256. |
| [8] | (c) Shi, X.; Wang, R.; Zeng, X.; Zeng, X.; Hu, H.; Xie, C.; Wang, M. Adv. Synth. Catal. 2018, 360, 4049. |
| [8] | (d) Wu, X.; Xiong, H.; Sun, S.; Cheng, J. Org. Lett. 2018, 20, 1396. |
| [8] | (e) Zheng, G.; Tian, M.; Xu, Y.; Xu, Y.; Li, X. Org. Chem. Front. 2018, 5, 998. |
| [8] | (f) Cai, L.; Zhu, X.; Zhu, X.; Lin, A.; Yao, H. Org. Chem. Front. 2019, 6, 3688. |
| [8] | (g) Xie, W.; Chen, X.; Shi, J.; Li, J.; Liu, R. Org. Chem. Front. 2019, 6, 2662. |
| [8] | (h) Dong, Y.; Yu, J.-T.; Sun, S.; Cheng, J. Chem. Commun. 2020, 56, 6688. |
| [8] | (i) Hong, C.; Jiang, X.; Yu, S.; Lu, Z.; Zhang, Y. Chin. J. Org. Chem. 2021, 41, 888 (in Chinese). |
| [8] | (洪超, 蒋希程, 于书玲, 刘占祥, 张玉红, 有机化学, 2021, 41, 888.) |
| [8] | (j) Lee, S. C.; Son, J.-Y.; Kim, J. Y.; Eom, H.; Jang, S. B.; Lee, P. H. Adv. Synth. Catal. 2021, 363, 512. |
| [8] | (k) Aher, Y. N.; Pawar, A. B. J. Org. Chem. 2022, 87, 12608. |
| [8] | (l) Caiuby, C. A. D.; Furniel, L. G.; Burtoloso, A. C. B. Chem. Sci. 2022, 13, 1192. |
| [8] | (m) Kumar, S.; Nunewar, S.; Sabbi, T. K.; Kanchupalli, V. Org. Lett. 2022, 24, 3395. |
| [8] | (n) Ming, S.; Yang, J.; Wu, S.; Yao, G.; Xiong, H.; Du, Y.; Gong, J. Org. Chem. Front. 2022, 9, 5147. |
| [8] | (o) Zheng, Y.-C.; Shu, B.; Zeng, Y.-F.; Chen, S.-Y.; Song, J.-L.; Liu, Y.-Z.; Xiao, L.; Liu, X.-G.; Zhang, X.; Zhang, S.-S. Org. Chem. Front. 2022, 9, 5185. |
| [8] | (p) Phukon, J.; Bhorali, P.; Changmai, S.; Gogoi, S. Org. Lett. 2023, 25, 215. |
| [9] | (a) Johnson, B. M.; Shu, Y.-Z.; Zhuo, X.; Meanwell, N. A. J. Med. Chem. 2020, 63, 6315. |
| [9] | (b) Mandal, D.; Maji, S.; Pal, T.; Sinha, S. K.; Maiti, D. Chem. Commun. 2022, 58, 10442. |
| [9] | (c) Chen, D.; Jiang, J.; Wan, J.-P. Chin. J. Chem. 2022, 40, 2582. |
| [10] | Ma, R.; Deng, Z.; Wang, K.; Huang, D.; Hu, Y.; Lü, X. Chin. J. Org. Chem. 2022, 42, 353 (in Chinese). |
| [10] | (马然松, 邓周斌, 王克虎, 黄丹凤, 胡雨来, 闾肖波, 有机化学, 2022, 42, 353.) |
| [11] | (a) Wen, S.; Tian, Q.; Chen, Y.; Zhang, Y.; Cheng, G. Org. Lett. 2021, 23, 7407. |
| [11] | (b) Wen, S.; Chen, Y.; Tian, Q.; Zhang, Y.; Cheng, G. J. Org. Chem. 2022, 87, 1124. |
| [11] | (c) Wen, S.; Zhang, Y.; Tian, Q.; Chen, Y.; Cheng, G. Org. Chem. Front. 2022, 9, 4388. |
| [12] | (a) Pan, M.; Tong, Y.; Qiu, X.; Zeng, X.; Xiong, B. Chem. Commun. 2022, 58, 12443. |
| [12] | (b) Sun, Y.; Yang, Z.; Lu, S.-N.; Chen, Z.; Wu, X.-F. Org. Lett. 2022, 24, 6822. |
| [12] | (c) Yang, Z.; Tang, J.; Chen, Z.; Wu, X.-F. Org. Lett. 2022, 24, 7288. |
| [12] | (d) Yang, D.; Wang, Y.; Wang, T.; Dou, Q.; Zhou, K.; Zhai, H.; Cheng, B. Adv. Synth. Catal. 2023, 365, 88. |
| [12] | (e) Sun, Y.; Ling, S.; Duan, Y.; Li, J.; Zhengkai, C.; Wu, X.-F. Adv. Synth. Catal. 2023, 365, 1521. |
| [12] | (f) Wei, G.; Sun, Y.; Zheng, D.; Qiu, S.; Chen, Z.; Wu, X.-F. Eur. J. Org. Chem. 2023, 26, e202300090. |
| [13] | (a) Wen, S.; Lv, W.; Ba, D.; Liu, J.; Cheng, G. Chem. Sci. 2019, 10, 9104. |
| [13] | (b) Yu, J.; Wen, S.; Ba, D.; Lv, W.; Chen, Y.; Cheng, G. Org. Lett. 2019, 21, 6366. |
| [13] | (c) Wen, S.; Chen, Y.; Zhao, Z.; Ba, D.; Lv, W.; Cheng, G. J. Org. Chem. 2020, 85, 1216. |
| [14] | For the selected papers on Rh(III)-catalyzed C—H activations, see: (a) Fu, L.; Xu, W.; Pu, M.; Wu, Y.-D.; Liu, Y.; Wan, J.-P. Org. Lett. 2022, 24, 3003. |
| [14] | (b) Chen, D.; Zhou, L.; Liu, Y.; Wan, J.-P. Chem. Commun. 2023, 59, 4036. |
| [14] | (c) Chen, D.; Zhou, L.; Wen, C.; Wan, J.-P. J. Org. Chem. 2023, 88, 8619 |
| [14] | (d) Cheng, G.-L.; Wen, S.; Zhang, S.-S. CN 202211461998, 2022. |
| [14] | (e) Zhang, Y.; Ling, S.; Li, P.; Chen, Z.; Wu, X.-F. Org. Lett. 2022, 24, 8864. |
| [15] | Wappes, E. A.; Nakafuku, K. M.; Nagib, D. A. J. Am. Chem. Soc. 2017, 139, 10204. |
| [16] | Lian, Y.; Coffey, S. B.; Li, Q.; Londregan, A. T. Org. Lett. 2016, 18, 1362. |
/
| 〈 |
|
〉 |