

氮杂环丙烷与不饱和化合物发生[3+2]扩环反应的研究进展
收稿日期: 2023-05-09
修回日期: 2023-07-11
网络出版日期: 2023-08-30
基金资助
国家自然科学基金(22171036); 国家自然科学基金(22007028); 河南省自然科学基金(232300421126); 河南师范大学化学化工学院开放研究基金(2020YB03)
Recent Progress on [3+2] Ring-Expansion Reaction of Aziridines with Unsaturated Compounds
Received date: 2023-05-09
Revised date: 2023-07-11
Online published: 2023-08-30
Supported by
National Natural Science Foundation of China(22171036); National Natural Science Foundation of China(22007028); Natural Science Foundation of Henan Province(232300421126); Open Research Fund of School of Chemistry and Chemical Engineering, Henan Normal University(2020YB03)
郝二军 , 丁笑波 , 王珂新 , 周红昊 , 杨启亮 , 石磊 . 氮杂环丙烷与不饱和化合物发生[3+2]扩环反应的研究进展[J]. 有机化学, 2023 , 43(12) : 4057 -4074 . DOI: 10.6023/cjoc202305008
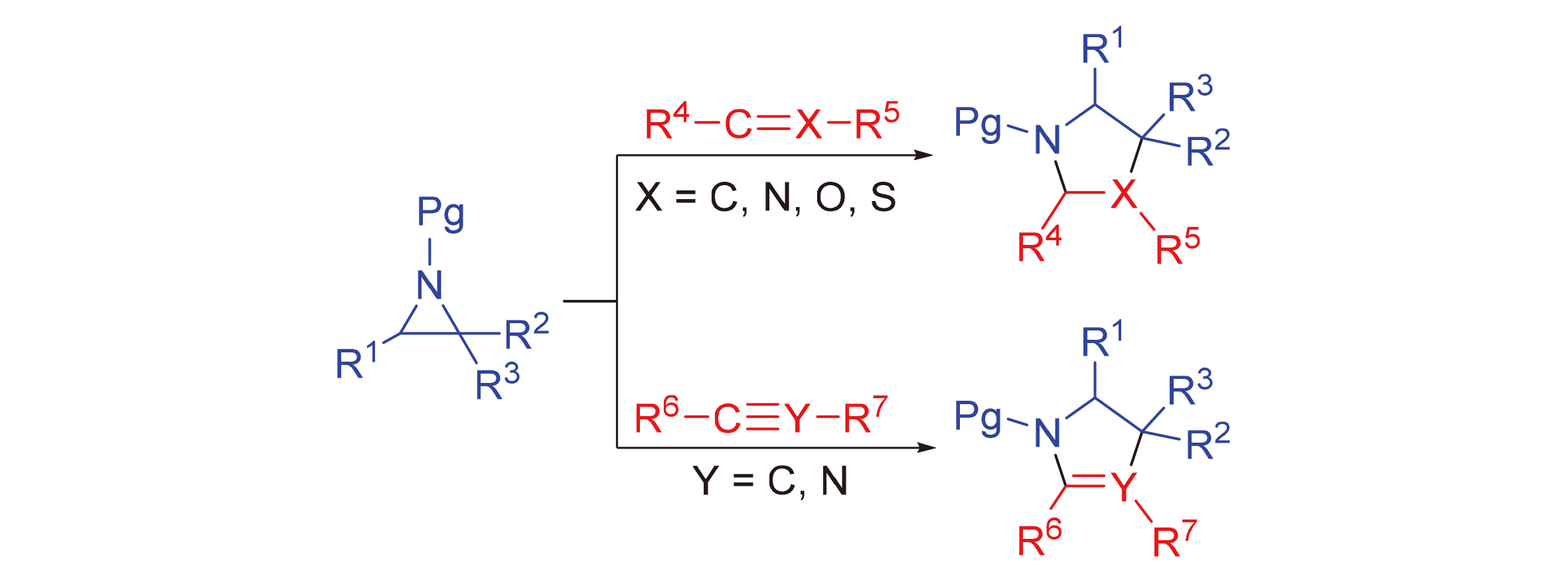
Due to their high ring strain, aziridines readily undergo ring-expansion reactions, reacting with various unsaturated compounds to form heterocyclic compounds. These heterocyclic compounds serve as important scaffolds for drugs, natural products, and bioactive molecules. Additionally, they are valuable organic intermediates with wide-ranging applications in medicine, agriculture, chemical engineering, organic synthesis, and related fields. In recent years, there has been a significant increase in the synthesis of heterocyclic compounds using aziridines as three-atom synthons, resulting in a rapid advancement of research in this area. This review aims to provide an overview of the most recent [3+2] ring-expansion reactions involving aziridines and unsaturated compounds, such as olefins, aldehydes, ketones, and nitriles, over the past decade. Furthermore, the prospect in this field is also discussed.
Key words: aziridine; heterocyclic compound; [3+2] ring-expansion reaction
| [1] | Ohno H. Chem. Rev. 2014, 114, 7784 |
| [2] | Ilardi E. A.; Njardarson J. T. J. Org. Chem. 2013, 78, 9533. |
| [3] | Wu Y.; Zhou X.; Xiao W.; Chen J. Chin. J. Org. Chem. 2020, 40, 3760. (in Chinese) |
| [3] | (吴雅莉, 周雪松, 肖文精, 陈加荣, 有机化学, 2020, 40, 3760.) |
| [4] | Du T.; Li S.; He Y.; Long H.; Liu X.; Li H. B.; Liu L. Chin. J. Chem. 2022, 40, 1681. |
| [5] | Xu B.; Zhang Z. M.; Han J.; Gu G.; Zhang J. Chin. J. Chem. 2022, 40, 1407. |
| [6] | Kini G. D.; Hennen W. J.; Robins R. K. J. Org. Chem. 1986, 51, 4436. |
| [7] | Arredondo V. M.; Tian S.; McDonald F. E.; Marks T. J. J. Am. Chem. Soc. 1999, 121, 3633. |
| [8] | Butler M. S. J. Nat. Prod. 2004, 67, 2141. |
| [9] | Magedov I. V.; Luchetti G.; Evdokimov N. M.; Manpadi M.; Steelant W. F. A.; Van slambrouck S.; Tongwa P.; Antipin M. Y.; Kornienko A. Bioorg. Med. Chem. Lett. 2008, 18, 1392. |
| [10] | More S. S.; Mohan T. K.; Kumar Y. S.; Kumar U. K.; Patel N. B. Beilstein J. Org. Chem. 2011, 7, 831. |
| [11] | Cai C.; Hu B.; Lü C. Chin. J. Org. Chem. 2005, 25, 1311. (in Chinese) |
| [11] | (蔡超君, 胡炳成, 吕春绪, 有机化学, 2005, 25, 1311.) |
| [12] | Wang Q.; Chang H.; Wei W.; Liu Q.; Gao W.; Li Y.; Li X. Chin. J. Org. Chem. 2016, 36, 939. (in Chinese) |
| [12] | (王清宇, 常宏宏, 魏文珑, 刘强, 高文超, 李彦威, 李兴, 有机化学, 2016, 36, 939.) |
| [13] | Gao Y.; Xiao Z.; Liu L.; Huang P. Chin. J. Org. Chem. 2017, 37, 1189. (in Chinese) |
| [13] | (高燕娇, 肖振华, 刘良先, 黄培强, 有机化学, 2017, 37, 1189.) |
| [14] | Feng J.-J.; Zhang J. ACS Catal. 2016, 6, 6651. |
| [15] | Lowe M. A.; Ostovar M.; Ferrini S.; Chen C. C.; Lawrence P. G.; Fontana F.; Calabrese A. A.; Aggarwal V. K. Angew. Chem., Int. Ed. 2011, 50, 6370. |
| [16] | Takahashi H.; Yasui S.; Tsunoi S.; Shibata I. Org. Lett. 2014, 16, 1192. |
| [17] | Xu C.-F.; Zheng B.-H.; Suo J.-J.; Ding C.-H.; Hou X.-L. Angew. Chem., Int. Ed. 2015, 54, 1604. |
| [18] | Huang Y.; Zheng C.; Pan L.; Jin Q.; Zhao G. J. Org. Chem. 2015, 80, 10710. |
| [19] | Lin T. Y.; Zhu C. Z.; Zhang P.; Wang Y.; Wu H. H.; Feng J. J.; Zhang J. Angew. Chem., Int. Ed. 2016, 55, 10844. |
| [20] | Feng J. J.; Lin T. Y.; Zhu C. Z.; Wang H.; Wu H. H.; Zhang J. J. Am. Chem. Soc. 2016, 138, 2178. |
| [21] | Wang B.; Liang M.; Tang J.; Deng Y.; Zhao J.; Sun H.; Tung C. H.; Jia J.; Xu Z. Org. Lett. 2016, 18, 4614. |
| [22] | Hao W.; Wu X.; Sun J. Z.; Siu J. C.; MacMillan S. N.; Lin S. J. Am. Chem. Soc. 2017, 139, 12141. |
| [23] | Rivinoja D. J.; Gee Y. S.; Gardiner M. G.; Ryan J. H.; Hyland C. J. T. ACS Catal. 2017, 7, 1053. |
| [24] | Zhu C.; Feng J.; Zhang J. Chin. J. Org. Chem. 2017, 37, 1165. (in Chinese) |
| [24] | (朱超泽, 冯见君, 张俊良, 有机化学, 2017, 37, 1165.) |
| [25] | Zhu C. Z.; Feng J. J.; Zhang J. Chem. Commun. 2018, 54, 2401. |
| [26] | Suo J. J.; Liu W.; Du J.; Ding C. H.; Hou X. L. Chem. Asian. J. 2018, 13, 959. |
| [27] | Zhang J. Q.; Tong F.; Sun B. B.; Fan W. T.; Chen J. B.; Hu D.; Wang X. W. J. Org. Chem. 2018, 83, 2882. |
| [28] | Wan S.-H.; Liu S.-T. Tetrahedron 2019, 75, 1166. |
| [29] | Xiao S.; Chen B.; Jiang Q.; He L.; Chu W.-D.; He C.-Y.; Liu Q.-Z. Org. Chem. Front. 2021, 8, 3729. |
| [30] | Zhu G. S.; Yang P. J.; Ma C. X.; Yang G.; Chai Z. Org. Lett. 2021, 23, 7933. |
| [31] | Dong P.; Chen L.; Yang Z.; Dong S.; Feng X. Org. Chem. Front. 2021, 8, 6874. |
| [32] | Shcherbakov N. V.; Titov G. D.; Chikunova E. I.; Filippov I. P.; Rostovskii N. V.; Kukushkin V. Y.; Dubovtsev A. Y. Org. Chem. Front. 2022, 9, 5133. |
| [33] | Griffin K.; Montagne C.; Hoang C. T.; Clarkson G. J.; Shipman M. Org. Biomol. Chem. 2012, 10, 1032. |
| [34] | Arena G.; Chen C. C.; Leonori D.; Aggarwal V. K. Org. Lett. 2013, 15, 4250. |
| [35] | Wang L.; Yang D.; Han F.; Li D.; Zhao D.; Wang R. Org. Lett. 2015, 17, 176. |
| [36] | Hashimoto T.; Takino K.; Hato K.; Maruoka K. Angew. Chem., Int. Ed. 2016, 55, 8081. |
| [37] | Cardoso A. L.; Henriques M. S.; Paixao J. A.; Pinho E. M. T. M. J. Org. Chem. 2016, 81, 9028. |
| [38] | Wang Y. N.; Li T. R.; Zhang M. M.; Cheng B. Y.; Lu L. Q.; Xiao W. J. J. Org. Chem. 2016, 81, 10491. |
| [39] | Liao Y.; Zhou B.; Xia Y.; Liu X.; Lin L.; Feng X. ACS Catal. 2017, 7, 3934. |
| [40] | Alajarin M.; Ba?on D.; Egea A.; Marín-Luna M.; Orenes R.-A.; Vidal A. Org. Chem. Front. 2018, 5, 2020. |
| [41] | Xing S.; Xia H.; Guo J.; Zou C.; Gao T.; Wang K.; Zhu B.; Pei M.; Bai M. J. Org. Chem. 2019, 84, 8984. |
| [42] | Rong J.; Jiang H.; Wang S.; Su Z.; Wang H.; Tao C. Org. Biomol. Chem. 2020, 18, 3149. |
| [43] | Zhao Q. Q.; Zhou X. S.; Xu S. H.; Wu Y. L.; Xiao W. J.; Chen J. R. Org. Lett. 2020, 22, 2470. |
| [44] | Li Y.; Chen F.; Zhu S.; Chu L. Org. Chem. Front. 2021, 8, 2196. |
| [45] | Viceriat A.; Marchand I.; Carret S.; Poisson J. F. Org. Lett. 2021, 23, 2449. |
| [46] | Wani I. A.; Sk S.; Mal A.; Sengupta A.; Ghorai M. K. Org. Lett. 2022, 24, 7867. |
| [47] | Xing S.; Wang Y.; Jin C.; Shi S.; Zhang Y.; Liao Z.; Wang K.; Zhu B. J. Org. Chem. 2022, 87, 6426. |
| [48] | Buchi G.; Rodriguez A. D.; Yakushijin K. J. Org. Chem. 1989, 54, 4494. |
| [49] | Wu X.; Zhang J. Synthesis 2012, 44, 2147. |
| [50] | Yoshiki M.; Ishibashi R.; Yamada Y.; Hanamoto T. Org. Lett. 2014, 16, 5509. |
| [51] | Spielmann K.; Lee A. V.; de Figueiredo R. M.; Campagne J. M. Org. Lett. 2018, 20, 1444. |
| [52] | Lin T. Y.; Wu H. H.; Feng J. J.; Zhang J. Org. Lett. 2018, 20, 3587. |
| [53] | Hajra S.; Abu Saleh S. K.; Hazra A.; Singh M. S. J. Org. Chem. 2019, 84, 8194. |
| [54] | Lemaire S.; Tulkens P. M.; Bambeke F. V. Antimicrob. Agents Chemother. 2010, 54, 2540. |
| [55] | Lin X.-Z.; Yang Z.-Z.; He L.-N.; Yuan Z.-Y. Green. Chem. 2015, 17, 795. |
| [56] | Adhikari D.; Miller A. W.; Baik M.-H.; Nguyen S. T. Chem. Sci. 2015, 6, 1293. |
| [57] | Punk M.; Merkley C.; Kennedy K.; Morgan J. B. ACS Catal. 2016, 6, 4694. |
| [58] | Zhou F.; Xie S.-L.; Gao X.-T.; Zhang R.; Wang C.-H.; Yin G.-Q.; Zhou J. Green Chem. 2017, 19, 3908. |
| [59] | Yang P. J.; Qi L.; Liu Z.; Yang G.; Chai Z. J. Am. Chem. Soc. 2018, 140, 17211. |
| [60] | Teranishi S.; Maeda K.; Kurahashi T.; Matsubara S. Org. Lett. 2019, 21, 2593. |
| [61] | Intrieri D.; Damiano C.; Sonzini P.; Gallo E. J. Porphyrins Phthalocyanines 2019, 23, 305. |
| [62] | Sonzini P.; Damiano C.; Intrieri D.; Manca G.; Gallo E. Adv. Synth. Catal. 2020, 362, 2961. |
| [63] | Seayad J.; Seayad A. M.; Ng J. K. P.; Chai C. L. L. ChemCatChem 2012, 4, 774. |
| [64] | Ueno A.; Kayaki Y.; Ikariya T. Green Chem. 2013, 15, 425. |
| [65] | Nale D. B.; Rana S.; Parida K.; Bhanage B. M. Appl. Catal., A 2014, 469, 340. |
| [66] | Chen W.; Zhong L.-X.; Peng X.-w.; Sun R.-C.; Lu F.-C. ACS Sustainable Chem. Eng. 2015, 3, 147. |
| [67] | Saptal V. B.; Bhanage B. M. ChemSusChem 2016, 9, 1980. |
| [68] | Liu A.-H.; Dang Y.-L.; Zhou H.; Zhang J.-J.; Lu X.-B. ChemCatChem 2018, 10, 2686. |
| [69] | Sengoden M.; North M.; Whitwood A. C. ChemSusChem 2019, 12, 3296. |
| [70] | Gerwick W. H.; Proteau P. J.; Nagle D. G.; Hamel E.; Blokhin A.; Slate D. L. J. Org. Chem. 1994, 59, 1243. |
| [71] | Boyce R. J.; Mulqueen G. C.; Pattenden G. Tetrahedron 1995, 51, 7321. |
| [72] | Hawkins C. J.; Lavin M. F.; Marshall K. A.; Van den Brenk A. L.; Watters D. J. J. Med. Chem. 1990, 33, 1634. |
| [73] | Zabriskie T. M.; Foster M. P.; Stout T. J.; Clardy J.; Ireland C. M. J. Am. Chem. Soc. 1990, 112, 8080. |
| [74] | Bhattacharyya A.; Kavitha C. V.; Ghorai M. K. J. Org. Chem. 2016, 81, 6433. |
| [75] | Sengoden M.; Irie R.; Punniyamurthy T. J. Org. Chem. 2016, 81, 11508. |
| [76] | Coin G.; Ferrier de Montal O.; Dubourdeaux P.; Latour J. M. Eur. J. Org. Chem. 2021, 2021, 443. |
/
| 〈 |
|
〉 |