

2β-Acetoxyferruginol去醋酸基骨架衍生物抑制α-葡萄糖苷酶活性研究
收稿日期: 2023-07-31
修回日期: 2023-10-10
网络出版日期: 2023-10-26
基金资助
广东省教育厅基金(2021KCXTD044); 广东省教育厅基金(2021KTSCX135)
α-Glucosidase Inhibition Research of Derivatives Based on 2β-Acetoxyferruginol Scaffold Excluding Acetic Acid Group
Received date: 2023-07-31
Revised date: 2023-10-10
Online published: 2023-10-26
Supported by
Funds of Department of Education of Guangdong Province(2021KCXTD044); Funds of Department of Education of Guangdong Province(2021KTSCX135)
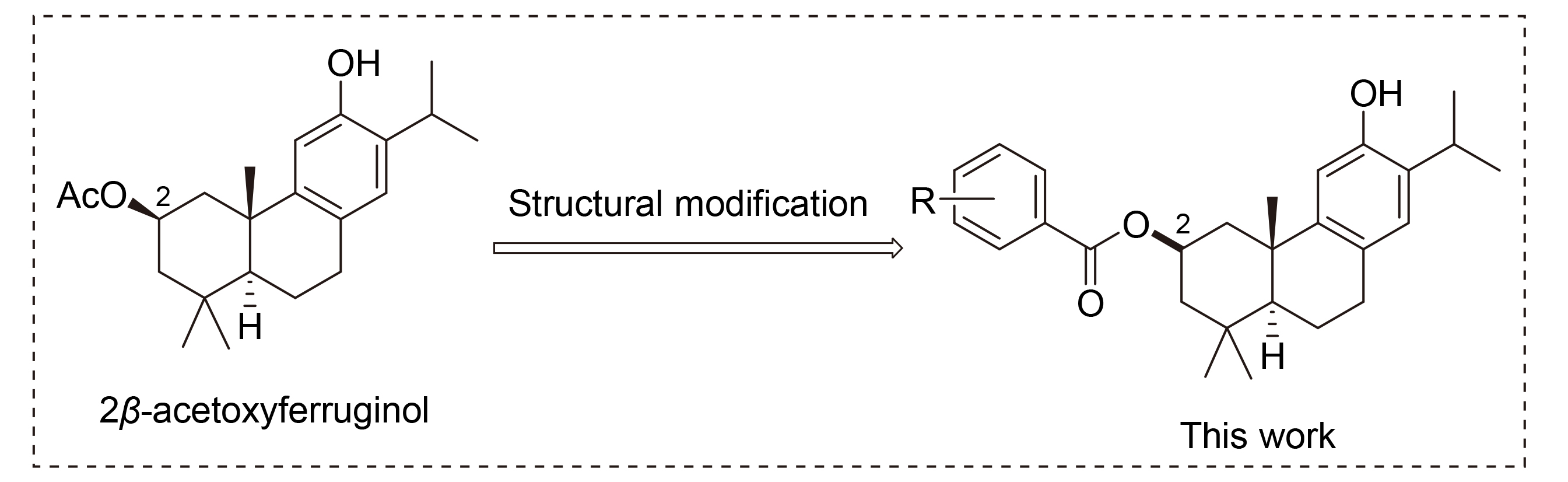
合成了系列2β-acetoxyferruginol去醋酸基骨架衍生物(1~24), 并测定了其α-葡萄糖苷酶抑制活性. 结果表明: 化合物1~24均有较好的α-葡萄糖苷酶抑制作用. 其中(3R,4aS,10aS)-6-羟基-1,1,4-三甲基-1,2,3,4,4a,9,10,10-八氢邻蒽- 3-基-4-(三氟甲基)苯甲酸酯(15)抑制α-葡萄糖苷酶活性最强[IC50=(23.91±2.34) μmol/L], 是阿卡波糖抑制活性的23.6倍. 构效关系分析表明三氟甲基的引入更有利于提高化合物的活性. 动力学结果显示化合物15为可逆非竞争性的α-葡萄糖苷酶抑制剂. 3D荧光结果表明化合物15与α-葡萄糖苷酶的结合可改变α-葡萄糖苷酶的构象. 分子对接结果显示化合物15与α-葡萄糖苷酶Asp68, Arg312, Tyr313形成了氢键, 与Phe177和Phe300形成疏水作用.
关键词: 2β-acetoxyferruginol; α-葡萄糖苷酶; 3D荧光; 分子对接
吴思敏 , 唐嘉欣 , 周于佳 , 徐学涛 , 张昊星 , 王少华 . 2β-Acetoxyferruginol去醋酸基骨架衍生物抑制α-葡萄糖苷酶活性研究[J]. 有机化学, 2024 , 44(2) : 613 -621 . DOI: 10.6023/cjoc202307027
In this study, a series derivatives based on 2β-acetoxyferruginol scaffold excluding acetic acid group (1~24) were synthesized and their inhibitory activities on α-glucosidase were determined. The results showed that all compounds 1~24 had good α-glucosidase inhibitory activities. Among them, (3R,4aS,10aS)-6-hydroxy-1,1,4a-trimethyl-1,2,3,4,4a,9,10,10a-octahy- drophenanthren-3-yl 4-(trifluoromethyl)benzoate (15) had the strongest inhibitory activity [IC50=(23.91±2.34) μmol/L], about 23.6-fold more active than acarbose. The structure activity relationship analysis showed that the introduction of trifluoromethyl was more conducive to enhance its activity. The kinetic results showed that compound 15 was a reversible and non competitive α-glucosidase inhibitor. The 3D fluorescence results indicated that the binding of α-glucosidase with compound 15 could change the conformation of α-glucosidase. The molecular docking results showed that compound 15 made hydrogen bonds with Asp68, Arg312, and Tyr313, and formed hydrophobic interactions with Phe177 and Phe300.

Key words: 2β-acetoxyferruginol; α-glucosidase; 3D fluorescence; molecular docking
| [1] | Rohm, T. V.; Meier, D. T.; Olefsky, J. M.; Donath, M. Y. Immunit. 2022, 55, 31. |
| [2] | Cole, J. B.; Florez, J. C. Nat. Rev. Nephrol. 2020, 16, 377. |
| [3] | Chiba S. Biosci. Biotechnol. Biochem. 1997, 61, 1233. |
| [4] | Zhang, Z.; Dai, C.; Wu, H.; Li, J.; Nan, F. Chin. J. Org. Chem. 2021, 41, 3204 (in Chinese). |
| [4] | (张正, 戴成球, 吴红红, 李静雅, 南发俊, 有机化学. 2021, 41, 3204.) |
| [5] | Kong, Y.; Yang, B.; Zhuang, Y.; Zhang, J.; Sun, D.; Dong, C. Chin. J. Org. Chem. 2022, 42, 770 (in Chinese). |
| [5] | (孔媛芳, 杨彬, 庄严, 张京玉, 孙德梅, 董春红, 有机化学. 2022, 42, 770.) |
| [6] | Liu, G.; Li, J.; Shi, L.; Liu, M.; Cai, B. Chin. J. Org. Chem. 2021, 41, 2974 (in Chinese). |
| [6] | (刘改枝, 李金鑫, 史礼君, 刘萌芽, 蔡邦荣, 有机化学. 2021, 41, 2974.) |
| [7] | Arunagirinathan, G.; Khwaja, N. U. D. Curr. Drug Saf. 2021, 16, 122. |
| [8] | Eguchi, K.; Komori, T.; Saito, T.; Hoshide, S.; Kario, K. J. Electrocardiol. 2018, 51, 21. |
| [9] | Lin, F.; Wang, Z.; Dai, D.; Zhou, X.; Wu, L. Chin. J. Org. Chem. 2022, 42, 1248 (in Chinese). |
| [9] | (林芳霞, 王治鸿, 代德财, 周学明, 吴禄勇, 有机化学. 2022, 42, 1248.) |
| [10] | Dirir, A. M.; Daou, M.; Yousef, A. F.; Yousef, L. F. Phytochem. Rev. 2022, 21, 1049. |
| [11] | Ichale, R.; Kanhed, A. M.; Vora, A. Mol. Divers. 2023, 23, 32013. |
| [12] | Kim S. D. Food Chem. 2013, 13, 297. |
| [13] | Lin, J.; Xiao, D.; Lu, L.; Liang, B.; Xiong, Z.; Xu, X. T. J. Mol. Struct. 2023, 34, 234. |
| [14] | Khan, H.; Zafar, M.; Patel, S.; Shah, S. M.; Bishayee, A. Food Chem. Toxicol. 2019, 130, 207. |
| [15] | Khan, S.; Ullah, H.; Rahim, F.; Nawaz, M.; Hussain, R.; Rasheed, L. J. Mol. Struct. 2022, 1269, 231. |
| [16] | Xu, X. T.; Deng, X. Y.; Chen, J.; Liang, Q. M.; Zhang, K.; Li, D. L.; Wu, P. P.; Zheng, X.; Zhou, R. P.; Jiang, Z. Y.; Ma, A. J.; Chen, W. H.; Wang, S. H. Eur. J. Med. Chem. 2020, 189, 45. |
| [17] | Zhang, X.; Zheng, Y. Y.; Hu, C. M.; Wu, X. Z.; Lin, J.; Xiong, Z.; Zhang, K.; Xu, X. T. Arab. J. Chem. 2022, 15, 231. |
| [18] | Fan, M. Y.; Yang, W.; Peng, Z. Y.; He, Y.; Wang G. C. Bioorg. Chem. 2023, 131, 456. |
| [19] | Zhong, X.; Yang, S.; Liu, T.; Ji, S.; Hu, J.; Li, H. Eur. J. Med. Chem. 2018, 150, 841. |
| [20] | Hu, C. M.; Wang, W. J.; Ye, Y. N.; Kang, Y.; Lin, J.; Wu, P. P.; Li, D. L.; Bai, L. P.; Xu, X. T.; Li, B. Q.; Zhang, K. Bioorg. Chem. 2021, 116, 105291. |
| [21] | Deng, X. Y.; Ke, J. J.; Zheng, Y. Y.; Li, D. L.; Zhang, K.; Zheng, X.; Wu, J. Y.; Xiong, Z.; Wu, P. P.; Xu, X. T. J. Enzyme Inhib. Med. Chem. 2022, 37, 451. |
| [22] | Xiao, D.; Lu, L.; Liang, B.; Xiong, Z.; Xu, X. T.; Chen, W. H. Eur. J. Med. Chem. 2023, 261, 115795. |
| [23] | Min, X.; Lu, L.; Xu, X.T.; Wen, Y.; Zheng, X. Int. J. Biol. Macromol. 2023, 253, 126962. |
| [24] | Wang, G.; Wang, J.; Xie, Z.; Chen, M.; Li, L.; Peng, Y.; Chen, S.; Li, W.; Deng, B. Bioorg. Chem. 2017, 72, 228. |
| [25] | Wang, G.; Peng, Z.; Wang, J.; Li, X.; Li, J. Eur. J. Med. Chem. 2017, 125, 423. |
| [26] | Wang, G.; Peng, Z.; Gong, Z.; Li, Y. Bioorg. Chem. 2018, 78, 195. |
| [27] | Leong, S. W.; Abas, F.; Lam, K. W.; Yusoff, K. Bioorg. Med. Chem. Lett. 2018, 28, 302. |
| [28] | Li, S.; Yin, L.; Yi, J.; Zhang, L. M.; Yang, L. J. Food Biochem. 2021, 45, e13550. |
| [29] | Narender, T.; Madhur, G.; Jaiswal, N.; Agrawal, M.; Maurya, C. K.; Rahuja, N.; Srivastava, A. K.; Tamrakar, A. K. Eur. J. Med. Chem. 2013, 63, 162. |
| [30] | Putta, S.; Sastry Yarla, N.; Kumar Kilari, E.; Surekha, C.; Aliev, G.; Basavaraju Divakara, M.; Sridhar Santosh, M.; Ramu, R.; Zameer, F.; Prasad, N.; Chintala, R. Curr. Top. Med. Chem. 2016, 16, 2532. |
| [31] | Wu, X.; Zhu, W.; Lu, L.; Hu, C.; Zheng, Y.; Zhang, X.; Lin, J.; Wu, J.; Xiong, Z.; Zhang, K.; Xu, X. Arab. J. Chem. 2023, 16, 104659. |
/
| 〈 |
|
〉 |