

锰催化环丁醇开环的C—C键氟化反应
收稿日期: 2024-01-26
修回日期: 2024-03-15
网络出版日期: 2024-03-28
基金资助
国家自然科学基金(92156008); 国家自然科学基金(22161142016); 山东省泰山学者计划和山东省自然科学基金(ZR2020QB018)
Manganese-Catalyzed Ring-Opening C—C Bond Fluorination of Cyclobutanols
Received date: 2024-01-26
Revised date: 2024-03-15
Online published: 2024-03-28
Supported by
National Natural Science Foundation of China(92156008); National Natural Science Foundation of China(22161142016); Taishan Scholar Program at Shandong Province and the Natural Science Foundation of Shandong Province(ZR2020QB018)
王丽梅 , 刘晓圆 , 昝金成 , 孙书涛 , 刘磊 , 李伟 , 刘希功 . 锰催化环丁醇开环的C—C键氟化反应[J]. 有机化学, 2024 , 44(7) : 2333 -2340 . DOI: 10.6023/cjoc202401031
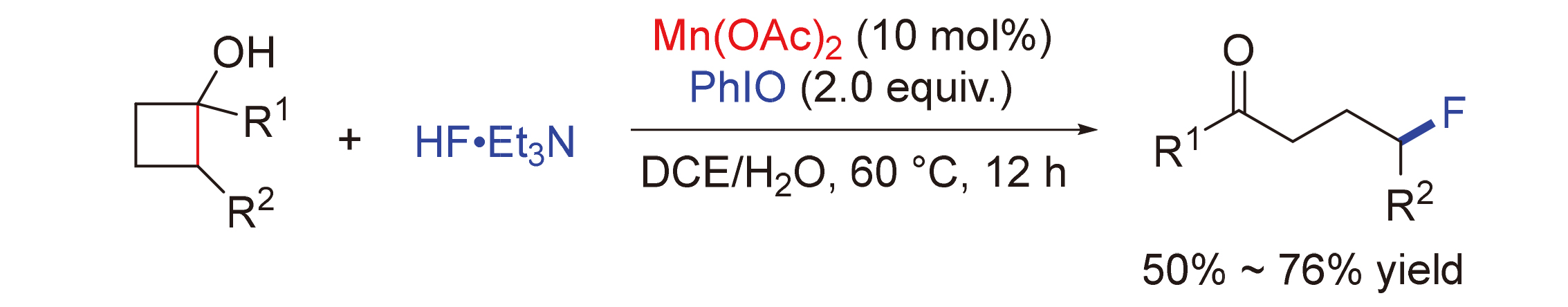
Manganese-catalyzed C—C bond cleavage of cyclobutanols has attracted great attention due to the high abundance and cheap and eco-friendly behaviour. A manganese-catalyzed ring-opening C—C bond fluorination of cyclobutanols is reported. Under mild conditions, the reaction provides a straightforward access to γ-fluorinated ketones using 10 mol% Mn(OAc)2 as catalyst and electrophilic fluorination reagent, which was generated in situ from HF•Et3N and PhIO, as fluorine source. The reaction has an excellent functional-group tolerance and displays a broad substrate scope, affording the corresponding products in 50%~76% yields.
| [1] | (a) Mu?ller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881. |
| [1] | (b) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320. |
| [1] | (c) O’Hagan, D. J. Fluorine Chem. 2010, 131, 1071. |
| [1] | (d) Johnson, B. M.; Shu, Y.-Z.; Zhuo, X.; Meanwell, N. A. J. Med. Chem. 2020, 63, 6315. |
| [1] | (e) Cheng, M.; Guo, C.; Gross, M. L. Angew. Chem., Int. Ed. 2020, 59, 5880. |
| [1] | (f) Ogawa, Y.; Tokunaga, E.; Kobayashi, O.; Hirai, K.; Shibata, N. iScience 2020, 23, 101467. |
| [1] | (g) Li, Y.; Dai, M.; Wang, L.; Wang, G. J. Ethnopharmacol. 2021, 269, 113749. |
| [1] | (h) Wang, L.; Wang, P.; Wang, D.; Tao, M.; Xu, W. Nat. Prod. Commun. 2020, 15, 20912088. |
| [1] | (i) Song, A.; Ding, T.; Wei, N.; Yang, J.; Ma, M.; Zheng, S.; Jin, H. Toxicol. Appl. Pharmacol. 2023, 472, 116574. |
| [2] | (a) Thayer, A. M. Chem. Eng. News 2006, 84, 15. |
| [2] | (b) Ametamey, S. M.; Honer, M.; Schubiger, P. A. Chem. Rev. 2008, 108, 1501. |
| [2] | (c) Littich, R.; Scott, P. J. H. Angew. Chem., Int. Ed. 2012, 51, 1106. |
| [2] | (d) Wang, J.; Sánchez-Roselló, M.; Ace?a, J. L.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432. |
| [2] | (e) de la Torre, B. G.; Albericio, F. Molecules 2021, 26, 627. |
| [2] | (f) Wang, L.; Wang, N.; Qi, Y.; Sun, S.; Liu, X.; Li, W.; Liu, L. Chin. J. Org. Chem. 2020, 40, 3934. (in Chinese) |
| [2] | (王琳, 王楠, 齐越, 孙书涛, 刘希功, 李伟, 刘磊, 有机化学, 2020, 40, 3934.) |
| [2] | (g) Wang, D.; Kan, L.; Ma, Y.; Liu, L. Chin. J. Org. Chem. 2021, 41, 3192. (in Chinese) |
| [2] | (王东琳, 阚玲珑, 马玉道, 刘磊, 有机化学, 2021, 41, 3192.) |
| [2] | (h) Du, T.; Li, S.; He, Y.; Long, H.; Liu, X.; Li, H.-B.; Liu, L. Chin. J. Chem. 2022, 40, 1681. |
| [2] | (i) Zang, L.; Xu, H.; Huang, C.; Wang, C.; Wang, R.; Chen, R.; Chen, Y.; Wang, L.; Wang, H. Recent Pat. Anticancer Drug Discov. 2022, 17, 145. |
| [2] | (j) Hong, H.; Zou, Q.; Liu, Y.; Wang, S.; Shen, G.; Yan, X. ChemMedChem 2021, 16, 2381. |
| [2] | (k) Wang, X.; Shen, C.; Wang, X.; Tang, J.; Wu, Z.; Huang, Y.; Shao, W.; Geng, K.; Xie, H.; Pu, Z. Chin. Med. 2023, 18, 112. |
| [3] | (a) O’Hagan, D. Chem. Soc. Rev. 2008, 37, 308. |
| [3] | (b) Furuya, T.; Kamlet, A. S.; Ritter, T. Nature 2011, 473, 470. |
| [3] | (c) Campbell, M. G.; Ritter, T. Chem. Rev. 2015, 115, 612. |
| [3] | (d) Champagne, P. A.; Desroches, J.; Hamel, J.-D.; Vandamme, M.; Paquin, J.-F. Chem. Rev. 2015, 115, 9073. |
| [3] | (e) Yerien, D. E.; Bonesi, S.; Postigo, A. Org. Biomol. Chem. 2016, 14, 8398. |
| [3] | (f) Zhu, Y.; Han, J.; Wang, J.; Shibata, N.; Sodeoka, M.; Soloshonok, V. A.; Coelho, J. A. S.; Toste, F. D. Chem. Rev. 2018, 118, 3887. |
| [3] | (g) Szpera, R.; Moseley, D. F. J.; Smith, L. B.; Sterling, A. J.; Gouverneur, V. Angew. Chem., Int. Ed. 2019, 58, 14824. |
| [4] | (a) Seiser, T.; Saget, T.; Tran, D. N.; Cramer, N. Angew. Chem., Int. Ed. 2011, 50, 7740. |
| [4] | (b) Souillart, L.; Parker, E.; Cramer, N. Top. Curr. Chem. 2014, 346, 163. |
| [4] | (c) Marek, I.; Masarwa, A.; Delaye, P.-O.; Leibeling, M. Angew. Chem., Int. Ed. 2015, 54, 414. |
| [4] | (d) Ren, R.; Zhu, C. Synlett 2016, 27, 1139. |
| [4] | (e) Wu, X.; Zhu, C. Chem. Rec. 2018, 18, 587. |
| [4] | (f) Yan, H.; Smith, G. S.; Chen, F.-E. Green Synth. Catal. 2022, 3, 219. |
| [4] | (g) Sun, Y.; Yang, L.; Cheng, Y.; An, G.; Li, G. Chin. Chem. Lett. 2024, 35, 109250. |
| [4] | (h) Zhai, S.; Qiu, S.; Yang, S.; Gao, X.; Feng, X.; Yun, C.; Han, N.; Niu, Y.; Wang, J.; Zhai, H. Chin. Chem. Lett. 2023, 34, 107657. |
| [4] | (i) Wu, K.; Li, X.; Wang, W.; Huang, Y.; Jiang, Q.; Li, W.; Chen, Y.; Yang, Y.; Li, C. ACS Catal. 2022, 12, 4261. |
| [4] | (j) Fang, L.; Jia, S.; Fan, S.; Zhu, J. Chem. Commun. 2023, 59, 10392. |
| [5] | (a) Zhao, H.; Fan, X.; Yu, J.; Zhu, C. J. Am. Chem. Soc. 2015, 137, 3490. |
| [5] | (b) Ren, S.; Feng, C.; Loh, T.-P. Org. Biomol. Chem. 2015, 13, 5105. |
| [5] | (c) Ishida, N.; Okumura, S.; Nakanishi, Y.; Murakami, M. Chem. Lett. 2015, 44, 821. |
| [6] | (a) Ren, R.; Wu, Z.; Xu, Y.; Zhu, C. Angew. Chem., Int. Ed. 2016, 55, 2866. |
| [6] | (b) Ren, R.; Wu, Z.; Zhu, C. Chem. Commun. 2016, 52, 8160. |
| [6] | (c) Wang, D.; Ren, R.; Zhu, C. J. Org. Chem. 2016, 81, 8043. |
| [6] | (d) Allen, B. D. W.; Hareram, M. D.; Seastram, A. C.; McBride, T.; Wirth, T.; Browne, D. L.; Morrill, L. C. Org. Lett. 2019, 21, 9241. |
| [6] | (e) Zhang, Y.-H.; Zhang, W.-W.; Zhang, Z.-Y.; Zhao, K.; Loh, T.-P. Org. Lett. 2019, 21, 5101. |
| [6] | (f) Meyer, T.; Yin, Z.; Wu, X.-F. Tetrahedron Lett. 2019, 60, 864. |
| [6] | (g) Lou, C.; Lv, L.; Li, Z. Adv. Synth. Catal. 2022, 364, 3743. |
| [6] | (h) He, Y.; Du, C.; Han, J.; Zhu, C.; Xie, J. Chin. J. Chem. 2022, 40, 1546. |
| [7] | Lu, Y.-C.; West, J. G. ACS Catal. 2021, 11, 12721. |
| [8] | (a) Kitamura, T.; Kuriki, S.; Morshed, M. H.; Hori, Y. Org. Lett. 2011, 13, 2392. |
| [8] | (b) Kitamura, T.; Muta, K. J. Org. Chem. 2014, 79, 5842. |
| [8] | (c) Kitamura, T.; Mizuno, S.; Muta, K.; Oyamada, J. J. Org. Chem. 2018, 83, 2773. |
| [8] | (d) Wang, L.; Kitamura, T.; Zhou, Y.; Butler, G.; Han, J.; Soloshonok, V. A. J. Fluorine Chem. 2020, 240, 109670. |
| [9] | (a) Ren, R.; Zhao, H.; Huan, L.; Zhu, C. Angew. Chem., Int. Ed. 2015, 54, 12692. |
| [9] | (b) Huan, L.; Zhu, C. Org. Chem. Front. 2016, 3, 146. |
| [9] | (c) Liu, W.; Huang, X.; Cheng, M.-J.; Nielsen, R. J.; Goddard, W. A., III; Groves, J. T. Science 2012, 337, 1322. |
| [9] | (d) Huang, X.; Liu, W.; Hooker, J. M.; Groves, J. T. Angew. Chem., Int. Ed. 2015, 54, 5241. |
| [10] | (a) He, Y.; Tian, C.; An, G.; Li, G. Chin. Chem. Lett. 2024, 35, 108546. |
| [10] | (b) Lv, X.; Cheng, Y.; Zong, Y.; Wang, Q.; An, G.; Wang, J.; Li, G. ACS Catal. 2023, 13, 7310. |
/
| 〈 |
|
〉 |