有机化学 ›› 2024, Vol. 44 ›› Issue (7): 2333-2340.DOI: 10.6023/cjoc202401031 上一篇 下一篇
研究论文
王丽梅a, 刘晓圆a, 昝金成c, 孙书涛b, 刘磊b,*( ), 李伟a,*(
), 李伟a,*( ), 刘希功b,*(
), 刘希功b,*( )
)
收稿日期:2024-01-26
修回日期:2024-03-15
发布日期:2024-03-28
作者简介:基金资助:
Limei Wanga, Xiaoyuan Liua, Jincheng Zanc, Shutao Sunb, Lei Liub( ), Wei Lia(
), Wei Lia( ), Xigong Liub(
), Xigong Liub( )
)
Received:2024-01-26
Revised:2024-03-15
Published:2024-03-28
Contact:
E-mail: About author:Supported by:文章分享
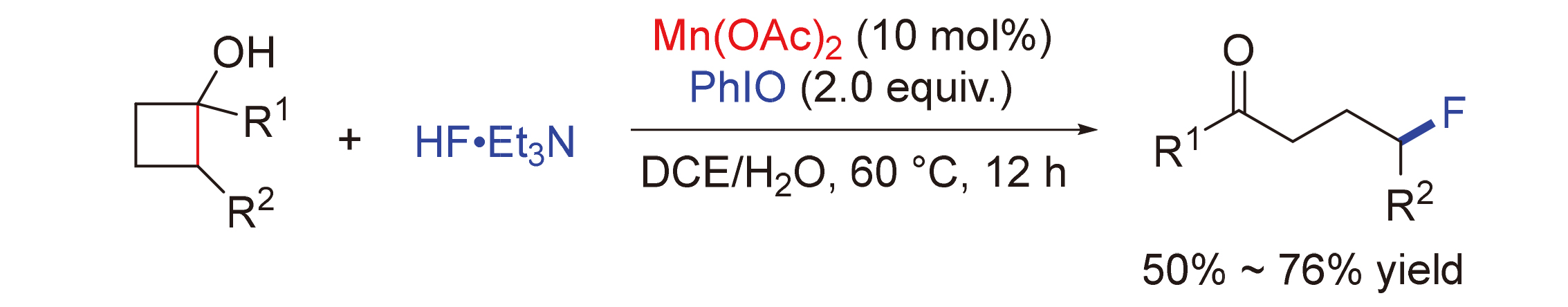
锰因其相对丰度高、价格低廉、环境友好等特点而在催化环丁醇的C—C键断裂官能化反应研究中备受关注. 报道了一例锰催化环丁醇开环C—C键氟化反应. 该反应在温和的条件下, 以10 mol%的Mn(OAc)2作为催化剂, HF•Et3N和PhIO作用原位生成的亲电氟化试剂作为氟源, 为直接合成γ-氟化酮提供了一种有效的途径. 该反应具有优异的官能团耐受性, 并显示出广泛的底物范围, 能够以50%~76%的产率得到相应的产物.
王丽梅, 刘晓圆, 昝金成, 孙书涛, 刘磊, 李伟, 刘希功. 锰催化环丁醇开环的C—C键氟化反应[J]. 有机化学, 2024, 44(7): 2333-2340.
Limei Wang, Xiaoyuan Liu, Jincheng Zan, Shutao Sun, Lei Liu, Wei Li, Xigong Liu. Manganese-Catalyzed Ring-Opening C—C Bond Fluorination of Cyclobutanols[J]. Chinese Journal of Organic Chemistry, 2024, 44(7): 2333-2340.
| Entry | Catalyst | Oxidant | Solvent | Temperature/℃ | Yieldb/% |
|---|---|---|---|---|---|
| 1 | Mn(OAc)2•4H2O | PhIO | DCE/H2O (V∶V=1∶1) | 60 | 61 |
| 2 | Mn(OAc)3 | PhIO | DCE/H2O (V∶V=1∶1) | 60 | 45 |
| 3 | Mn(OAc)2 | PhIO | DCE/H2O (V∶V=1∶1) | 60 | 76 |
| 4 | Mn(acac)2 | PhIO | DCE/H2O (V∶V=1∶1) | 60 | 36 |
| 5 | Mn(OAc)2 | PIDA | DCE/H2O (V∶V=1∶1) | 60 | 30 |
| 6 | Mn(OAc)2 | PIFA | DCE/H2O (V∶V=1∶1) | 60 | 25 |
| 7 | Mn(OAc)2 | IBX | DCE/H2O (V∶V=1∶1) | 60 | 35 |
| 8 | Mn(OAc)2 | Other oxidantc | DCE/H2O (V∶V=1∶1) | 60 | <20 |
| 9 | Mn(OAc)2 | PhIO | Acetonitrile/H2O (V∶V=1∶1) | 60 | 32 |
| 10 | Mn(OAc)2 | PhIO | Toluene/H2O (V∶V=1∶1) | 60 | <5 |
| 11 | Mn(OAc)2 | PhIO | THF/H2O (V∶V=1∶1) | 60 | <5 |
| 12 | Mn(OAc)2 | PhIO | DCE/H2O (V∶V=1∶2) | 60 | <5 |
| 13 | Mn(OAc)2 | PhIO | DCE/H2O (V∶V=2∶1) | 60 | <5 |
| 14 | Mn(OAc)2 | PhIO | DCE | 60 | <5 |
| 15 | Mn(OAc)2 | PhIO | DCE/H2O (V∶V=1∶1) | 25 | <5 |
| 16 | Mn(OAc)2 | PhIO | DCE/H2O (V∶V=1∶1) | 40 | 26 |
| 17 | Mn(OAc)2 | PhIO | DCE/H2O (V∶V=1∶1) | 50 | 29 |
| 18 | Mn(OAc)2 | PhIO | DCE/H2O (V∶V=1∶1) | 70 | 41 |
| Entry | Catalyst | Oxidant | Solvent | Temperature/℃ | Yieldb/% |
|---|---|---|---|---|---|
| 1 | Mn(OAc)2•4H2O | PhIO | DCE/H2O (V∶V=1∶1) | 60 | 61 |
| 2 | Mn(OAc)3 | PhIO | DCE/H2O (V∶V=1∶1) | 60 | 45 |
| 3 | Mn(OAc)2 | PhIO | DCE/H2O (V∶V=1∶1) | 60 | 76 |
| 4 | Mn(acac)2 | PhIO | DCE/H2O (V∶V=1∶1) | 60 | 36 |
| 5 | Mn(OAc)2 | PIDA | DCE/H2O (V∶V=1∶1) | 60 | 30 |
| 6 | Mn(OAc)2 | PIFA | DCE/H2O (V∶V=1∶1) | 60 | 25 |
| 7 | Mn(OAc)2 | IBX | DCE/H2O (V∶V=1∶1) | 60 | 35 |
| 8 | Mn(OAc)2 | Other oxidantc | DCE/H2O (V∶V=1∶1) | 60 | <20 |
| 9 | Mn(OAc)2 | PhIO | Acetonitrile/H2O (V∶V=1∶1) | 60 | 32 |
| 10 | Mn(OAc)2 | PhIO | Toluene/H2O (V∶V=1∶1) | 60 | <5 |
| 11 | Mn(OAc)2 | PhIO | THF/H2O (V∶V=1∶1) | 60 | <5 |
| 12 | Mn(OAc)2 | PhIO | DCE/H2O (V∶V=1∶2) | 60 | <5 |
| 13 | Mn(OAc)2 | PhIO | DCE/H2O (V∶V=2∶1) | 60 | <5 |
| 14 | Mn(OAc)2 | PhIO | DCE | 60 | <5 |
| 15 | Mn(OAc)2 | PhIO | DCE/H2O (V∶V=1∶1) | 25 | <5 |
| 16 | Mn(OAc)2 | PhIO | DCE/H2O (V∶V=1∶1) | 40 | 26 |
| 17 | Mn(OAc)2 | PhIO | DCE/H2O (V∶V=1∶1) | 50 | 29 |
| 18 | Mn(OAc)2 | PhIO | DCE/H2O (V∶V=1∶1) | 70 | 41 |
| [1] |
(a) Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881.
pmid: 31588625 |
|
(b) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320.
pmid: 31588625 |
|
|
(c) O’Hagan, D. J. Fluorine Chem. 2010, 131, 1071.
pmid: 31588625 |
|
|
(d) Johnson, B. M.; Shu, Y.-Z.; Zhuo, X.; Meanwell, N. A. J. Med. Chem. 2020, 63, 6315.
pmid: 31588625 |
|
|
(e) Cheng, M.; Guo, C.; Gross, M. L. Angew. Chem., Int. Ed. 2020, 59, 5880.
doi: 10.1002/anie.201907662 pmid: 31588625 |
|
|
(f) Ogawa, Y.; Tokunaga, E.; Kobayashi, O.; Hirai, K.; Shibata, N. iScience 2020, 23, 101467.
pmid: 31588625 |
|
|
(g) Li, Y.; Dai, M.; Wang, L.; Wang, G. J. Ethnopharmacol. 2021, 269, 113749.
pmid: 31588625 |
|
|
(h) Wang, L.; Wang, P.; Wang, D.; Tao, M.; Xu, W. Nat. Prod. Commun. 2020, 15, 20912088.
pmid: 31588625 |
|
|
(i) Song, A.; Ding, T.; Wei, N.; Yang, J.; Ma, M.; Zheng, S.; Jin, H. Toxicol. Appl. Pharmacol. 2023, 472, 116574.
pmid: 31588625 |
|
| [2] |
(a) Thayer, A. M. Chem. Eng. News 2006, 84, 15.
pmid: 22213395 |
|
(b) Ametamey, S. M.; Honer, M.; Schubiger, P. A. Chem. Rev. 2008, 108, 1501.
doi: 10.1021/cr0782426 pmid: 22213395 |
|
|
(c) Littich, R.; Scott, P. J. H. Angew. Chem., Int. Ed. 2012, 51, 1106.
doi: 10.1002/anie.201106785 pmid: 22213395 |
|
|
(d) Wang, J.; Sánchez-Roselló, M.; Aceña, J. L.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432.
pmid: 22213395 |
|
|
(e) de la Torre, B. G.; Albericio, F. Molecules 2021, 26, 627.
pmid: 22213395 |
|
|
(f) Wang, L.; Wang, N.; Qi, Y.; Sun, S.; Liu, X.; Li, W.; Liu, L. Chin. J. Org. Chem. 2020, 40, 3934. (in Chinese)
pmid: 22213395 |
|
|
(王琳, 王楠, 齐越, 孙书涛, 刘希功, 李伟, 刘磊, 有机化学, 2020, 40, 3934.)
doi: 10.6023/cjoc202004027 pmid: 22213395 |
|
|
(g) Wang, D.; Kan, L.; Ma, Y.; Liu, L. Chin. J. Org. Chem. 2021, 41, 3192. (in Chinese)
pmid: 22213395 |
|
|
(王东琳, 阚玲珑, 马玉道, 刘磊, 有机化学, 2021, 41, 3192.)
doi: 10.6023/cjoc202104003 pmid: 22213395 |
|
|
(h) Du, T.; Li, S.; He, Y.; Long, H.; Liu, X.; Li, H.-B.; Liu, L. Chin. J. Chem. 2022, 40, 1681.
pmid: 22213395 |
|
|
(i) Zang, L.; Xu, H.; Huang, C.; Wang, C.; Wang, R.; Chen, R.; Chen, Y.; Wang, L.; Wang, H. Recent Pat. Anticancer Drug Discov. 2022, 17, 145.
pmid: 22213395 |
|
|
(j) Hong, H.; Zou, Q.; Liu, Y.; Wang, S.; Shen, G.; Yan, X. ChemMedChem 2021, 16, 2381.
pmid: 22213395 |
|
|
(k) Wang, X.; Shen, C.; Wang, X.; Tang, J.; Wu, Z.; Huang, Y.; Shao, W.; Geng, K.; Xie, H.; Pu, Z. Chin. Med. 2023, 18, 112.
pmid: 22213395 |
|
| [3] |
(a) O’Hagan, D. Chem. Soc. Rev. 2008, 37, 308.
pmid: 29608052 |
|
(b) Furuya, T.; Kamlet, A. S.; Ritter, T. Nature 2011, 473, 470.
pmid: 29608052 |
|
|
(c) Campbell, M. G.; Ritter, T. Chem. Rev. 2015, 115, 612.
doi: 10.1021/cr500366b pmid: 29608052 |
|
|
(d) Champagne, P. A.; Desroches, J.; Hamel, J.-D.; Vandamme, M.; Paquin, J.-F. Chem. Rev. 2015, 115, 9073.
pmid: 29608052 |
|
|
(e) Yerien, D. E.; Bonesi, S.; Postigo, A. Org. Biomol. Chem. 2016, 14, 8398.
pmid: 29608052 |
|
|
(f) Zhu, Y.; Han, J.; Wang, J.; Shibata, N.; Sodeoka, M.; Soloshonok, V. A.; Coelho, J. A. S.; Toste, F. D. Chem. Rev. 2018, 118, 3887.
doi: 10.1021/acs.chemrev.7b00778 pmid: 29608052 |
|
|
(g) Szpera, R.; Moseley, D. F. J.; Smith, L. B.; Sterling, A. J.; Gouverneur, V. Angew. Chem., Int. Ed. 2019, 58, 14824.
pmid: 29608052 |
|
| [4] |
(a) Seiser, T.; Saget, T.; Tran, D. N.; Cramer, N. Angew. Chem., Int. Ed. 2011, 50, 7740.
pmid: 25266824 |
|
(b) Souillart, L.; Parker, E.; Cramer, N. Top. Curr. Chem. 2014, 346, 163.
doi: 10.1007/128_2013_505 pmid: 25266824 |
|
|
(c) Marek, I.; Masarwa, A.; Delaye, P.-O.; Leibeling, M. Angew. Chem., Int. Ed. 2015, 54, 414.
doi: 10.1002/anie.201405067 pmid: 25266824 |
|
|
(d) Ren, R.; Zhu, C. Synlett 2016, 27, 1139.
pmid: 25266824 |
|
|
(e) Wu, X.; Zhu, C. Chem. Rec. 2018, 18, 587.
pmid: 25266824 |
|
|
(f) Yan, H.; Smith, G. S.; Chen, F.-E. Green Synth. Catal. 2022, 3, 219.
pmid: 25266824 |
|
|
(g) Sun, Y.; Yang, L.; Cheng, Y.; An, G.; Li, G. Chin. Chem. Lett. 2024, 35, 109250.
pmid: 25266824 |
|
|
(h) Zhai, S.; Qiu, S.; Yang, S.; Gao, X.; Feng, X.; Yun, C.; Han, N.; Niu, Y.; Wang, J.; Zhai, H. Chin. Chem. Lett. 2023, 34, 107657.
pmid: 25266824 |
|
|
(i) Wu, K.; Li, X.; Wang, W.; Huang, Y.; Jiang, Q.; Li, W.; Chen, Y.; Yang, Y.; Li, C. ACS Catal. 2022, 12, 4261.
pmid: 25266824 |
|
|
(j) Fang, L.; Jia, S.; Fan, S.; Zhu, J. Chem. Commun. 2023, 59, 10392.
pmid: 25266824 |
|
| [5] |
(a) Zhao, H.; Fan, X.; Yu, J.; Zhu, C. J. Am. Chem. Soc. 2015, 137, 3490.
|
|
(b) Ren, S.; Feng, C.; Loh, T.-P. Org. Biomol. Chem. 2015, 13, 5105.
|
|
|
(c) Ishida, N.; Okumura, S.; Nakanishi, Y.; Murakami, M. Chem. Lett. 2015, 44, 821.
|
|
| [6] |
(a) Ren, R.; Wu, Z.; Xu, Y.; Zhu, C. Angew. Chem., Int. Ed. 2016, 55, 2866.
|
|
(b) Ren, R.; Wu, Z.; Zhu, C. Chem. Commun. 2016, 52, 8160.
|
|
|
(c) Wang, D.; Ren, R.; Zhu, C. J. Org. Chem. 2016, 81, 8043.
|
|
|
(d) Allen, B. D. W.; Hareram, M. D.; Seastram, A. C.; McBride, T.; Wirth, T.; Browne, D. L.; Morrill, L. C. Org. Lett. 2019, 21, 9241.
|
|
|
(e) Zhang, Y.-H.; Zhang, W.-W.; Zhang, Z.-Y.; Zhao, K.; Loh, T.-P. Org. Lett. 2019, 21, 5101.
|
|
|
(f) Meyer, T.; Yin, Z.; Wu, X.-F. Tetrahedron Lett. 2019, 60, 864.
doi: 10.1016/j.tetlet.2019.02.028 |
|
|
(g) Lou, C.; Lv, L.; Li, Z. Adv. Synth. Catal. 2022, 364, 3743.
|
|
|
(h) He, Y.; Du, C.; Han, J.; Zhu, C.; Xie, J. Chin. J. Chem. 2022, 40, 1546.
|
|
| [7] |
Lu, Y.-C.; West, J. G. ACS Catal. 2021, 11, 12721.
|
| [8] |
(a) Kitamura, T.; Kuriki, S.; Morshed, M. H.; Hori, Y. Org. Lett. 2011, 13, 2392.
doi: 10.1021/ol200632d pmid: 29431440 |
|
(b) Kitamura, T.; Muta, K. J. Org. Chem. 2014, 79, 5842.
doi: 10.1021/jo500691b pmid: 29431440 |
|
|
(c) Kitamura, T.; Mizuno, S.; Muta, K.; Oyamada, J. J. Org. Chem. 2018, 83, 2773.
doi: 10.1021/acs.joc.7b03223 pmid: 29431440 |
|
|
(d) Wang, L.; Kitamura, T.; Zhou, Y.; Butler, G.; Han, J.; Soloshonok, V. A. J. Fluorine Chem. 2020, 240, 109670.
pmid: 29431440 |
|
| [9] |
(a) Ren, R.; Zhao, H.; Huan, L.; Zhu, C. Angew. Chem., Int. Ed. 2015, 54, 12692.
|
|
(b) Huan, L.; Zhu, C. Org. Chem. Front. 2016, 3, 146.
|
|
|
(c) Liu, W.; Huang, X.; Cheng, M.-J.; Nielsen, R. J.; Goddard, W. A., III; Groves, J. T. Science 2012, 337, 1322.
|
|
|
(d) Huang, X.; Liu, W.; Hooker, J. M.; Groves, J. T. Angew. Chem., Int. Ed. 2015, 54, 5241.
|
|
| [10] |
(a) He, Y.; Tian, C.; An, G.; Li, G. Chin. Chem. Lett. 2024, 35, 108546.
|
|
(b) Lv, X.; Cheng, Y.; Zong, Y.; Wang, Q.; An, G.; Wang, J.; Li, G. ACS Catal. 2023, 13, 7310.
|
| [1] | 邹震雷, 李和寅, 黄梦君, 沈胤朴, 刘继阳, 王之兆, 张为钢, 王毅, 潘毅. 关于惰性含氟温室气体转化与利用的研究进展[J]. 有机化学, 2024, 44(6): 1831-1852. |
| [2] | 李洋, 董亚楠, 李跃辉. 经由N-硼基酰胺中间体的酰胺高效转化合成腈类化合物[J]. 有机化学, 2024, 44(2): 638-643. |
| [3] | 金玉坤, 任保轶, 梁福顺. 可见光介导的三氟甲基的选择性C-F键断裂及其在偕二氟类化合物合成中的应用[J]. 有机化学, 2024, 44(1): 85-110. |
| [4] | 朱传涛, 王松, 赵一凡, Herdewijn Piet, 刘丰五. 新型2,2'-缩水-L-苏糖嘧啶膦酸核苷的合成[J]. 有机化学, 2023, 43(9): 3167-3173. |
| [5] | 张晓雨, 李欣燕, 崔冰, 邵志晖, 赵铭钦. 四氢-β-咔啉衍生物的设计、合成及抗氧化性能研究[J]. 有机化学, 2023, 43(8): 2885-2894. |
| [6] | 安大列, 包志鹏, 吴小锋. 含碳氟类底物参与的羰基化反应研究进展[J]. 有机化学, 2023, 43(7): 2304-2312. |
| [7] | 曾成富, 何媛, 李清, 董琳. Ir(III)催化新型三组分串联三氟乙氧基化反应并一锅法构建复杂酰胺化合物[J]. 有机化学, 2023, 43(3): 1115-1123. |
| [8] | 刘桂杰, 付正强, 陈飞, 徐彩霞, 李敏, 刘宁. N-杂环卡宾-吡啶锰配合物/四丁基碘化铵催化CO2和环氧化物合成环状碳酸酯[J]. 有机化学, 2023, 43(2): 629-635. |
| [9] | 张豪, 赵庆彬, 阮忠睿, 刘振兴. 硅醚与硫(VI)氟化合物SuFEx点击反应进展[J]. 有机化学, 2023, 43(10): 3569-3579. |
| [10] | 孙奇, 孙泽颖, 俞泽, 王光伟. 镍催化炔烃的立体选择性芳基-二氟烷基化反应[J]. 有机化学, 2022, 42(8): 2515-2520. |
| [11] | 李响, 张依凡, 陆凯琳, 刘石惠, 张永强. 基于莪术醇胺氟化结构修饰的三维天然产物片段库的构建[J]. 有机化学, 2022, 42(7): 2124-2133. |
| [12] | 冉龙玉, 张成潘. 三氟甲磺酸三氟甲酯的反应研究进展[J]. 有机化学, 2022, 42(7): 2045-2054. |
| [13] | 李玉东, 李莹, 董亚楠, 夏春谷, 李跃辉. 锰催化的碳酸乙烯亚乙酯对喹唑啉酮的C—H烯丙基化[J]. 有机化学, 2022, 42(3): 847-853. |
| [14] | 郭檬檬, 于子伦, 陈玉兰, 葛丹华, 马猛涛, 沈志良, 褚雪强. 二氟烯醇硅醚作为含氟砌块在构建有机氟化物中的研究进展[J]. 有机化学, 2022, 42(11): 3562-3587. |
| [15] | 刘颖杰, 韩莹徽, 林立青, 许颖. 电化学催化下的多氟烷基化反应研究进展[J]. 有机化学, 2021, 41(3): 934-946. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||