

25-羟基胆固醇及其类似物的合成与抗神经炎活性研究
收稿日期: 2023-12-03
修回日期: 2024-02-27
网络出版日期: 2024-04-10
基金资助
国家自然科学基金(81960638); 国家自然科学基金(82160656); 国家自然科学基金(82360695)
Synthesis and Anti-neuroinflammatory Activities of 25-Hydroxycholesterol and Its Analogues
Received date: 2023-12-03
Revised date: 2024-02-27
Online published: 2024-04-10
Supported by
National Natural Science Foundation of China(81960638); National Natural Science Foundation of China(82160656); National Natural Science Foundation of China(82360695)
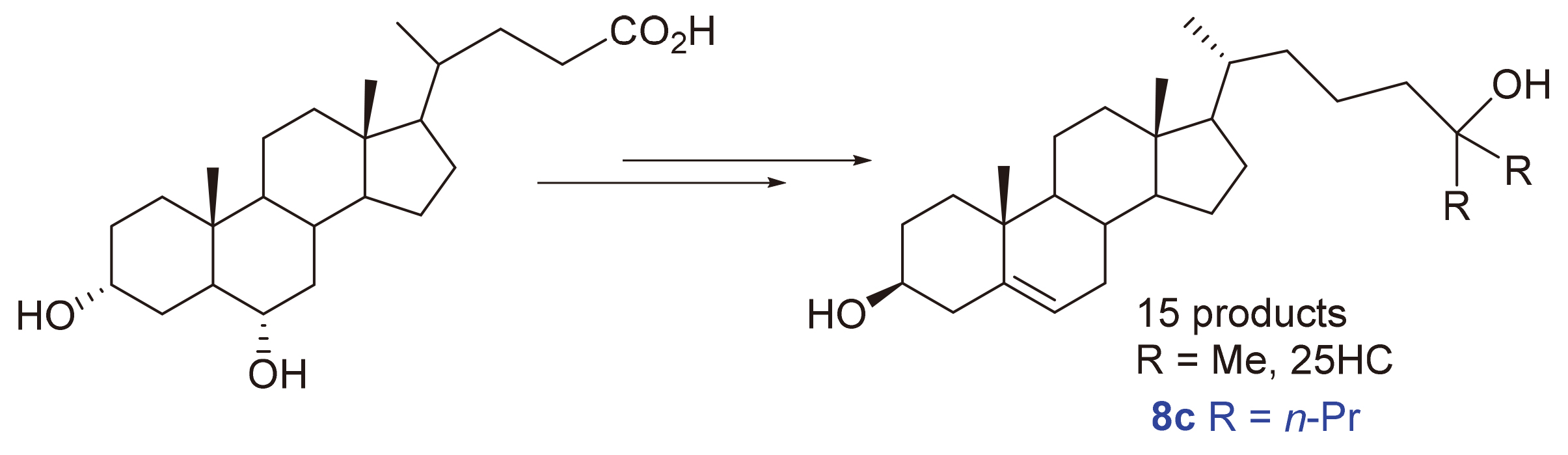
25-羟基胆固醇(25HC)具有许多重要的生物活性, 也是合成骨化二醇、骨化三醇等药物的关键中间体, 开发其原料易得、工艺简单以及易于放大的新合成路线具有重要意义. 以廉价易得的猪去氧胆酸为原料, 经羟基酯化、脱羧碘代、与α-酮酸酯反应进而水解脱羧、羟基磺酸酯化及消除, 最后与格氏试剂反应共6步反应合成了3个25-羟基胆固醇及其类似物, 总产率为20.8%~23.0%. 新路线操作简单、易于放大, 有望进一步发展成工业化合成路线. 通过改变酮酸酯及格氏试剂可方便地得到不同的25-羟基胆固醇类似物. 合成所得到的15个化合物中, 有9个是新的化合物. 对15个化合物进行生物活性研究, 结果表明, 26,27-二乙基胆甾-5-烯-3β,25-二醇(8c)具有良好的抗神经炎活性, 可通过抑制核转录因子(NF-κB)和丝裂原活化蛋白激酶(MAPK/JNK)信号通路, 抑制LPS(脂多糖)诱导的小鼠小胶质瘤细胞(BV-2)细胞释放NO、下调白细胞介素6 (IL-6)、肿瘤坏死因子α (TNF-α)、白细胞介素1β (IL-1β)等炎症因子的信使核糖核酸(mRNA)水平, 表明8c是有潜力的抗神经炎症的化合物.
关键词: 25-羟基胆固醇; 25-羟基胆固醇类似物; 合成; 抗神经炎活性
蓝柳淞 , 杨倩 , 李永怡 , 方淑君 , 黄宇轩 , 苏骏成 , 潘成学 , 苏桂发 . 25-羟基胆固醇及其类似物的合成与抗神经炎活性研究[J]. 有机化学, 2024 , 44(7) : 2305 -2314 . DOI: 10.6023/cjoc202312002
25-Hydroxycholesterol (25HC) has many important biological activities and is also a key intermediate for the synthesis of calcifediol, calcitriol, etc. It is of great significance to develop a new synthetic route which is easy to available raw materials, simple process, and easy to scale up. In this report, three 25-hydroxycholesterol and its analogues were synthesized from hyodeoxycholic acid via esterification of hydroxyl groups, decarboxylation iodization of carboxyl groups, ketonic hydrolysis after nucleophilic substitution with α-ketoester, sulfonate esterification then elimination, and finally reaction with Grignard reagent. The total yield was 20.8%~23.0%. The new route is simple to operate and easy to scale up, and is expected to be further developed into an industrial synthetic route. Similar analogues of 25-hydroxycholesterol can be easily prepared by changing α-ketoesters and Grignard reagents. Among the 15 compounds synthesized, there are 9 new compounds. Their biological activities were evaluated. The results showed that 26,27-diethylcholest-5-ene-3β,25-diol (8c) has good anti-neuritis activity and can inhibit NO release from LPS (lipopolysaccharides)-induced BV-2 (mouse microglia) cells by inhibiting Nuclear Factor kappa-B (NF-κB) and Mitogen-activated protein kinase/c-Jun N-terminal Kinase (MAPK/JNK) signaling pathways, and down-regulating mRNA-levels of inflammatory cytokines such as Interleukin-6 (IL-6), Tumor Necrosis Factor-α (TNF-α) and Interleukin-1β (IL-1β), indicating that 8c is a potential anti-neuroinflammatory compound.

| [1] | Cyster, J. G.; Dang, E. V.; Reboldi, A.; Yi, T.-S. Nat. Rev. Immunol. 2014, 14, 731. |
| [2] | Adams, C. M.; Reitz, J.; De Brabander, J. K.; Feramisco, J. D.; Li, L.; Brown, M. S.; Goldstein, J. L. J. Biol. Chem. 2004, 279, 52772. |
| [3] | Cashikar, A. G.; Toral-Rios, D.; Timm, D.; Romero, J.; Strickland, M.; Long, J. M.; Han, X.-L.; Holtzman, D. M.; Paul, S. M. J. Lipid Res. 2023, 64, 100350. |
| [4] | a) Reboldi, A.; Dang, E. V.; McDonald, J. G.; Liang, G.-S.; Russell, D. W.; Cyster, J. G. Science 2014, 345, 679. |
| [4] | (b) Jang, J.; Park, S.; Hur, H. J.; Cho, H.-J.; Hwang, I.; Kang, Y. P.; Im, I.; Lee, H.; Lee, E.; Yang, W.; Kang, H.-C.; Kwon, S. W.; Yu, J.-W.; Kim, D.-W. Nat. Commun. 2016, 7, 13129. |
| [4] | (c) Civra, A.; Cagno, V.; Donalisio, M.; Biasi, F.; Leonarduzzi, G.; Poli, G.; Lembo, D. Sci. Rep. 2014, 4, 7487. |
| [5] | a) Liu, S.-Y.; Aliyari, R.; Chikere, K.; Li, G.-M.; Marsden, M.D.; Smith, J. K.; Pernet, O.; Guo, H.-T.; Nusbaum, R.; Zack, J. A.; Freiberg, A. N.; Su, L.-S.; Lee, B.; Cheng, G.-H. Immunity 2013, 38, 92. |
| [5] | (b) Mao, S.; Ren, J.; Xu, Y.; Lin, J.; Pan, C.; Meng, Y.; Xu, N. Eur. J. Pharmacol. 2022, 926, 175033. |
| [5] | (c) Chen, Y.; Wang, S.; Yi, Z.; Tian, H.; Aliyari, R.; Li, Y.; Chen, G.; Liu, P.; Zhong, J.; Chen, X.; Du, P.; Su, L.; Qin, F. X.-F.; Deng, H.; Cheng, G. Sci. Rep. 2014, 4, 7242. |
| [6] | a) Lu, Z.; McBrearty, N.; Chen, J.-Y.; Tomar, V. S.; Zhang, H.-R.; De Rosa, G.; Tan, A.-W.; Weljie, A. M.; Beiting, D. P.; Miao, Z.; George, S. S.; Berger, A.; Saggu, G.; Diehl, J. A.; Koumenis, C.; Fuchs, S. Y. Cell Metab. 2022, 34, 1342. |
| [6] | (b) You, J.-S.; Lim, H.; Kim, T.-H.; Oh, J.-S.; Lee, G.-J.; Seo, Y.-S.; Kim, D. K.; Yu, S.-K.; Kim, H.-J.; Kim, C. S.; Kim, J.-S. Anticancer Res. 2020, 40, 779 |
| [7] | a) Cao, Q.; Luo, J.; Xiong, Y.; Liu, Z.-Z.; Ye, Q.-F. Int. Immunopharmacol. 2021, 96, 107643. |
| [7] | (b) Lv, S.-Y.; Ju, C.-H.; Peng, J.-T.; Liang, M.-L.; Zhu, F.; Wang, C.; Huang, K.; Cheng, M.; Zhang, F.-X. Int. J. Biol. Sci. 2020, 16, 298. |
| [8] | Zhang, L.; Sun, B.; Su, W.-K.; Jin, C. Chin. Pharm. J. 2019, 50, 695 (in Chinese). |
| [8] | (张凉, 孙彬, 苏为科, 金灿, 中国医药工业杂志, 2019, 50, 695.) |
| [9] | Zhao, Q.; Ji, L.; Qian, G.-P.; Liu, J.-G.; Wang, Z.-Q.; Yu, W.-F.; Chen, X.-Z. Steroids 2014, 85, 1. |
| [10] | a) Ogawa, S.; Kakiyama, G.; Muto, A.; Hosoda, A.; Mitamura, K.; Ikegawa, S.; Hofmann, A. F.; Iida, T. Steroids 2009, 74, 81. |
| [10] | (b) Wang, J.-T.; Chen, W.-Z.; Xia, J.; Zhao, P.; Xie, J.; Tang, L. Chem. React. 2017, 39, 668 (in Chinese). |
| [10] | (王建塔, 陈文章, 夏晶, 赵平, 谢珺, 汤磊, 化学试剂, 2017, 39, 668.) |
| [11] | a) Miyamoto, K.; Kubodera, N.; Murayama, E.; Ochi, K.; Mori, T.; Matsunaga, I. Synth. Commun. 1986, 16, 513. |
| [11] | (b) Brownholland, D. P.; Covey, D. F. Steroids 2017, 121, 22. |
| [12] | a) Liu, S.; Wang, G.-Q.; Liu, Z.-K.; Wang, Q.-A. Chin. J. Org. Chem. 2013, 33, 2216 (in Chinese). |
| [12] | (刘双, 汪钢强, 刘张坤, 汪秋安, 有机化学, 2013, 33, 2216.) |
| [12] | (b) Wang, Z.-Q.; Jiang, L.-Z.; Rong, S.-H. Chin. J. Med. Chem. 1994, 4, 89 (in Chinese). |
| [12] | (王钟麒, 姜立中, 容士宏, 中国药物化学杂志, 1994, 4, 89.) |
| [12] | (c) Wang, Z.-Q.; Yuan, B.-F. Acta Chim. Sinica 1994, 52, 403 (in Chinese). |
| [12] | (王钟麒, 阮奔放, 化学学报, 1994, 52, 403.) |
| [12] | (d) Ruan, B.-F.; Wang, Z.-Q. Acta Chim. Sinica 1993, 51, 612 (in Chinese). |
| [12] | (阮奔放, 王钟麒, 化学学报, 1993, 51, 612.) |
| [12] | (e) Wang, Z.-Q.; Jiang, L.-Z.; Zhou, W.-S.; Gong, Y.-M.; Qian, Y. Sci. China, Ser. B: Chem., ife Sci., Earth Sci. 1991, 34, 680 (in Chinese). |
| [12] | (王钟麒, 姜立中, 周维善, 龚逸民, 钱涌, 中国科学, |
| [12] | (B辑, 化学. 生命科学, 地学), 1991, 34, 680.) |
| [12] | (f) Wang, Z.-Q.; Jiang, L.-Z.; Zhou, W.-S. Acta Chim. Sinica 1990, 48, 99 (in Chinese). |
| [12] | (王钟麒, 姜立中, 周维善, 化学学报, 1990, 48, 99.) |
| [12] | (g) Zhou, W.-S.; Wang, Z.-Q.; Jiang, B. Acta Chim. Sinica 1988, 46, 1150 (in Chinese). |
| [12] | (周维善, 王钟麒, 姜标, 化学学报, 1988, 46, 1150.) |
| [13] | a) Peng, Y.-H.; Wen, Y.-C.; Chen, Y.-Q. Acta Chim. Sinica 1985, 43, 698 (in Chinese). |
| [13] | (彭逸华, 温业淳, 陈毓群, 化学学报, 1985, 43, 698.) |
| [13] | (b) Wang, Z.-Q.; Jiang, L.-Z.; Zhou, W.-S. Chin. Chem. Lett. 1992, 3, 409. |
| [13] | (c) Jin, C.; Wang, Y.-L.; Sun, B.; Su, W.-K. J. Chem. Res. 2018, 42, 96. |
| [14] | Nakai, Y.; Moriyama, K.; Togo, H. Eur. J. Org. Chem. 2016, 2016, 768. |
| [15] | a) Tan, X. Q.; Song, T.; Wang, Z. T.; Chen, H.; Cui, L.; Li, C. Z. Org. Lett. 2017, 19, 1634. |
| [15] | (b) Wang, Z. T.; Zhu, L.; Yin, F.; Su, Z. Q.; Li, Z. D.; Li, C. Z. J. Am. Chem. Soc. 2012, 134, 425. |
| [16] | Camps, P.; Lukach, A. E.; Pujol, X.; Vazquez, S. Tetrahedron 2000, 56, 2703. |
| [17] | Candish, L.; Standley, E. A.; Gomez-Suarez, A.; Mukherjee, S.; Glorius, F.Chem.-Eur. J. 2016, 22, 9971. |
| [18] | Partridge, J. J.; Faber, S.; Uskokovic, M. R. Helv. Chim. Acta 1974, 57, 84. |
| [19] | Xiao, J.; Song, J.-Y.; Lin, B.; Li, W.; Yang, Y.-Q.; Liu, J.-Y.; Hou, Y.; Chen, G.; Li, N. J. Nat. Prod. 2020, 83, 864. |
| [20] | Liu, Y.-C.; Feng, N.; Li, W.-W.; Tu, P.-F.; Chen, J.-P.; Han, J.-Y.; Zeng, K.-W. Molecules 2020, 25, 2840. |
| [21] | Eguchi, T.; Sai, H.; Takatsuto, S.; Hara, N.; Ikekawa, N. Chem. Pharm. Bull. 1988, 36, 2303. |
/
| 〈 |
|
〉 |