有机化学 ›› 2024, Vol. 44 ›› Issue (7): 2305-2314.DOI: 10.6023/cjoc202312002 上一篇 下一篇
研究论文
蓝柳淞, 杨倩, 李永怡, 方淑君, 黄宇轩, 苏骏成*( ), 潘成学*, 苏桂发*
), 潘成学*, 苏桂发*
收稿日期:2023-12-03
修回日期:2024-02-27
发布日期:2024-07-25
作者简介:基金资助:
Liusong Lan, Qian Yang, Yongyi Li, Shujun Fang, Yuxuan Huang, Juncheng Su( ), Chengxue Pan, Guifa Su
), Chengxue Pan, Guifa Su
Received:2023-12-03
Revised:2024-02-27
Published:2024-07-25
Contact:
E-mail: About author:Supported by:文章分享
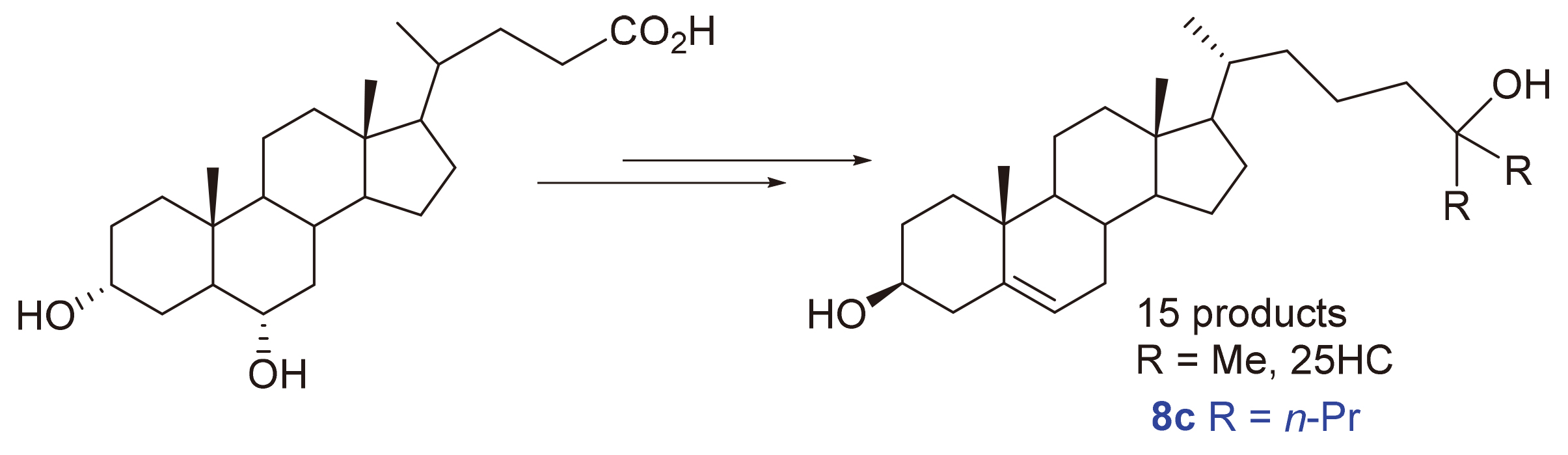
25-羟基胆固醇(25HC)具有许多重要的生物活性, 也是合成骨化二醇、骨化三醇等药物的关键中间体, 开发其原料易得、工艺简单以及易于放大的新合成路线具有重要意义. 以廉价易得的猪去氧胆酸为原料, 经羟基酯化、脱羧碘代、与α-酮酸酯反应进而水解脱羧、羟基磺酸酯化及消除, 最后与格氏试剂反应共6步反应合成了3个25-羟基胆固醇及其类似物, 总产率为20.8%~23.0%. 新路线操作简单、易于放大, 有望进一步发展成工业化合成路线. 通过改变酮酸酯及格氏试剂可方便地得到不同的25-羟基胆固醇类似物. 合成所得到的15个化合物中, 有9个是新的化合物. 对15个化合物进行生物活性研究, 结果表明, 26,27-二乙基胆甾-5-烯-3β,25-二醇(8c)具有良好的抗神经炎活性, 可通过抑制核转录因子(NF-κB)和丝裂原活化蛋白激酶(MAPK/JNK)信号通路, 抑制LPS(脂多糖)诱导的小鼠小胶质瘤细胞(BV-2)细胞释放NO、下调白细胞介素6 (IL-6)、肿瘤坏死因子α (TNF-α)、白细胞介素1β (IL-1β)等炎症因子的信使核糖核酸(mRNA)水平, 表明8c是有潜力的抗神经炎症的化合物.
蓝柳淞, 杨倩, 李永怡, 方淑君, 黄宇轩, 苏骏成, 潘成学, 苏桂发. 25-羟基胆固醇及其类似物的合成与抗神经炎活性研究[J]. 有机化学, 2024, 44(7): 2305-2314.
Liusong Lan, Qian Yang, Yongyi Li, Shujun Fang, Yuxuan Huang, Juncheng Su, Chengxue Pan, Guifa Su. Synthesis and Anti-neuroinflammatory Activities of 25-Hydroxycholesterol and Its Analogues[J]. Chinese Journal of Organic Chemistry, 2024, 44(7): 2305-2314.
| Compd. | Survival rate/% | Compd. | Survival rate/% |
|---|---|---|---|
| 4a | 102.64±5.45 | 6c | 121.65±3.41*** |
| 4b | 3.84±0.36**** | 7a | 120.48±3.71*** |
| 4c | 3.26±0.04**** | 7b | 98.38±4.58 |
| 5a | 54.69±17.96* | 7c | 118.12±3.15*** |
| 5b | 4.06±0.57**** | 8a | 27.52±0.69**** |
| 5c | 4.12±0.51**** | 8b | 23.97±3.76**** |
| 6a | 104.09±4.36 | 8c | 112.82±8.91 |
| 6b | 120.31±4.51** | Control | 100±2.56 |
| HDCA | 102.18±8.06 | DEX | 85.05±2.84*** |
| Compd. | Survival rate/% | Compd. | Survival rate/% |
|---|---|---|---|
| 4a | 102.64±5.45 | 6c | 121.65±3.41*** |
| 4b | 3.84±0.36**** | 7a | 120.48±3.71*** |
| 4c | 3.26±0.04**** | 7b | 98.38±4.58 |
| 5a | 54.69±17.96* | 7c | 118.12±3.15*** |
| 5b | 4.06±0.57**** | 8a | 27.52±0.69**** |
| 5c | 4.12±0.51**** | 8b | 23.97±3.76**** |
| 6a | 104.09±4.36 | 8c | 112.82±8.91 |
| 6b | 120.31±4.51** | Control | 100±2.56 |
| HDCA | 102.18±8.06 | DEX | 85.05±2.84*** |
| Compd. | Release of nitric oxide/% | Compd. | Release of nitric oxide/% |
|---|---|---|---|
| 4a | 76.18±13.74* | 7a | 59.70±8.25** |
| 6a | 61.16±11.65** | 7b | 61.16±5.53*** |
| 6b | 45.78±23.93* | 7c | 71.78±8.25** |
| 6c | 39.55±16.41** | 8c | 12.98±6.31**** |
| Control | 12.82±8.25 | DEX | 30.03±8.32*** |
| LPS | 100±7.20#### | HDCA | 56.62±6.42 |
| Compd. | Release of nitric oxide/% | Compd. | Release of nitric oxide/% |
|---|---|---|---|
| 4a | 76.18±13.74* | 7a | 59.70±8.25** |
| 6a | 61.16±11.65** | 7b | 61.16±5.53*** |
| 6b | 45.78±23.93* | 7c | 71.78±8.25** |
| 6c | 39.55±16.41** | 8c | 12.98±6.31**** |
| Control | 12.82±8.25 | DEX | 30.03±8.32*** |
| LPS | 100±7.20#### | HDCA | 56.62±6.42 |
| Compd. | Survival rate/% | Compd. | Survival rate/% |
|---|---|---|---|
| 4a | 43.54±2.98**** | 7a | 1.90±0.12**** |
| 6a | 25.64±2.75**** | 7b | 1.33±0.41**** |
| 6b | 26.14±5.74**** | 7c | 1.60±0.21**** |
| 6c | 6.47±1.30**** | 8c | 56.22±1.30**** |
| Control | 100±1.19 | DEX | 14.37±2.93**** |
| LPS | 88.57±2.01 |
| Compd. | Survival rate/% | Compd. | Survival rate/% |
|---|---|---|---|
| 4a | 43.54±2.98**** | 7a | 1.90±0.12**** |
| 6a | 25.64±2.75**** | 7b | 1.33±0.41**** |
| 6b | 26.14±5.74**** | 7c | 1.60±0.21**** |
| 6c | 6.47±1.30**** | 8c | 56.22±1.30**** |
| Control | 100±1.19 | DEX | 14.37±2.93**** |
| LPS | 88.57±2.01 |
| Compd. | Control | LPS | Release of nitric oxide/% | IC50/(μmol•L-1) | |||
|---|---|---|---|---|---|---|---|
| 6.25 μmol•L-1 | 12.50 μmol•L-1 | 25.00 μmol•L-1 | 50.00 μmol•L-1 | ||||
| 8c | 10.68±4.56 | 100±11.10 | 63.81±5.74 | 39.69±7.72 | 17.29±4.75 | 11.26±5.47 | 9.55 |
| DEX | 10.68±4.56 | 100±11.10 | — | — | — | 58.36±4.97 | — |
| Compd. | Control | LPS | Release of nitric oxide/% | IC50/(μmol•L-1) | |||
|---|---|---|---|---|---|---|---|
| 6.25 μmol•L-1 | 12.50 μmol•L-1 | 25.00 μmol•L-1 | 50.00 μmol•L-1 | ||||
| 8c | 10.68±4.56 | 100±11.10 | 63.81±5.74 | 39.69±7.72 | 17.29±4.75 | 11.26±5.47 | 9.55 |
| DEX | 10.68±4.56 | 100±11.10 | — | — | — | 58.36±4.97 | — |




| [1] |
Cyster, J. G.; Dang, E. V.; Reboldi, A.; Yi, T.-S. Nat. Rev. Immunol. 2014, 14, 731.
|
| [2] |
Adams, C. M.; Reitz, J.; De Brabander, J. K.; Feramisco, J. D.; Li, L.; Brown, M. S.; Goldstein, J. L. J. Biol. Chem. 2004, 279, 52772.
|
| [3] |
Cashikar, A. G.; Toral-Rios, D.; Timm, D.; Romero, J.; Strickland, M.; Long, J. M.; Han, X.-L.; Holtzman, D. M.; Paul, S. M. J. Lipid Res. 2023, 64, 100350.
|
| [4] |
a) Reboldi, A.; Dang, E. V.; McDonald, J. G.; Liang, G.-S.; Russell, D. W.; Cyster, J. G. Science 2014, 345, 679.
doi: 10.1126/science.1254790 pmid: 25104388 |
|
(b) Jang, J.; Park, S.; Hur, H. J.; Cho, H.-J.; Hwang, I.; Kang, Y. P.; Im, I.; Lee, H.; Lee, E.; Yang, W.; Kang, H.-C.; Kwon, S. W.; Yu, J.-W.; Kim, D.-W. Nat. Commun. 2016, 7, 13129.
pmid: 25104388 |
|
|
(c) Civra, A.; Cagno, V.; Donalisio, M.; Biasi, F.; Leonarduzzi, G.; Poli, G.; Lembo, D. Sci. Rep. 2014, 4, 7487.
pmid: 25104388 |
|
| [5] |
a) Liu, S.-Y.; Aliyari, R.; Chikere, K.; Li, G.-M.; Marsden, M.D.; Smith, J. K.; Pernet, O.; Guo, H.-T.; Nusbaum, R.; Zack, J. A.; Freiberg, A. N.; Su, L.-S.; Lee, B.; Cheng, G.-H. Immunity 2013, 38, 92.
doi: 10.1016/j.immuni.2012.11.005 pmid: 23273844 |
|
(b) Mao, S.; Ren, J.; Xu, Y.; Lin, J.; Pan, C.; Meng, Y.; Xu, N. Eur. J. Pharmacol. 2022, 926, 175033.
pmid: 23273844 |
|
|
(c) Chen, Y.; Wang, S.; Yi, Z.; Tian, H.; Aliyari, R.; Li, Y.; Chen, G.; Liu, P.; Zhong, J.; Chen, X.; Du, P.; Su, L.; Qin, F. X.-F.; Deng, H.; Cheng, G. Sci. Rep. 2014, 4, 7242.
pmid: 23273844 |
|
| [6] |
a) Lu, Z.; McBrearty, N.; Chen, J.-Y.; Tomar, V. S.; Zhang, H.-R.; De Rosa, G.; Tan, A.-W.; Weljie, A. M.; Beiting, D. P.; Miao, Z.; George, S. S.; Berger, A.; Saggu, G.; Diehl, J. A.; Koumenis, C.; Fuchs, S. Y. Cell Metab. 2022, 34, 1342.
|
|
(b) You, J.-S.; Lim, H.; Kim, T.-H.; Oh, J.-S.; Lee, G.-J.; Seo, Y.-S.; Kim, D. K.; Yu, S.-K.; Kim, H.-J.; Kim, C. S.; Kim, J.-S. Anticancer Res. 2020, 40, 779
|
|
| [7] |
a) Cao, Q.; Luo, J.; Xiong, Y.; Liu, Z.-Z.; Ye, Q.-F. Int. Immunopharmacol. 2021, 96, 107643.
|
|
(b) Lv, S.-Y.; Ju, C.-H.; Peng, J.-T.; Liang, M.-L.; Zhu, F.; Wang, C.; Huang, K.; Cheng, M.; Zhang, F.-X. Int. J. Biol. Sci. 2020, 16, 298.
|
|
| [8] |
Zhang, L.; Sun, B.; Su, W.-K.; Jin, C. Chin. Pharm. J. 2019, 50, 695 (in Chinese).
|
|
(张凉, 孙彬, 苏为科, 金灿, 中国医药工业杂志, 2019, 50, 695.)
|
|
| [9] |
Zhao, Q.; Ji, L.; Qian, G.-P.; Liu, J.-G.; Wang, Z.-Q.; Yu, W.-F.; Chen, X.-Z. Steroids 2014, 85, 1.
doi: 10.1016/j.steroids.2014.02.002 pmid: 24582707 |
| [10] |
a) Ogawa, S.; Kakiyama, G.; Muto, A.; Hosoda, A.; Mitamura, K.; Ikegawa, S.; Hofmann, A. F.; Iida, T. Steroids 2009, 74, 81.
|
|
(b) Wang, J.-T.; Chen, W.-Z.; Xia, J.; Zhao, P.; Xie, J.; Tang, L. Chem. React. 2017, 39, 668 (in Chinese).
|
|
|
(王建塔, 陈文章, 夏晶, 赵平, 谢珺, 汤磊, 化学试剂, 2017, 39, 668.)
|
|
| [11] |
a) Miyamoto, K.; Kubodera, N.; Murayama, E.; Ochi, K.; Mori, T.; Matsunaga, I. Synth. Commun. 1986, 16, 513.
pmid: 28300584 |
|
(b) Brownholland, D. P.; Covey, D. F. Steroids 2017, 121, 22.
doi: S0039-128X(17)30037-5 pmid: 28300584 |
|
| [12] |
a) Liu, S.; Wang, G.-Q.; Liu, Z.-K.; Wang, Q.-A. Chin. J. Org. Chem. 2013, 33, 2216 (in Chinese).
|
|
(刘双, 汪钢强, 刘张坤, 汪秋安, 有机化学, 2013, 33, 2216.)
|
|
|
(b) Wang, Z.-Q.; Jiang, L.-Z.; Rong, S.-H. Chin. J. Med. Chem. 1994, 4, 89 (in Chinese).
|
|
|
(王钟麒, 姜立中, 容士宏, 中国药物化学杂志, 1994, 4, 89.)
|
|
|
(c) Wang, Z.-Q.; Yuan, B.-F. Acta Chim. Sinica 1994, 52, 403 (in Chinese).
|
|
|
(王钟麒, 阮奔放, 化学学报, 1994, 52, 403.)
|
|
|
(d) Ruan, B.-F.; Wang, Z.-Q. Acta Chim. Sinica 1993, 51, 612 (in Chinese).
|
|
|
(阮奔放, 王钟麒, 化学学报, 1993, 51, 612.)
|
|
|
(e) Wang, Z.-Q.; Jiang, L.-Z.; Zhou, W.-S.; Gong, Y.-M.; Qian, Y. Sci. China, Ser. B: Chem., ife Sci., Earth Sci. 1991, 34, 680 (in Chinese).
|
|
|
(王钟麒, 姜立中, 周维善, 龚逸民, 钱涌, 中国科学,
|
|
|
(B辑, 化学. 生命科学, 地学), 1991, 34, 680.)
|
|
|
(f) Wang, Z.-Q.; Jiang, L.-Z.; Zhou, W.-S. Acta Chim. Sinica 1990, 48, 99 (in Chinese).
|
|
|
(王钟麒, 姜立中, 周维善, 化学学报, 1990, 48, 99.)
|
|
|
(g) Zhou, W.-S.; Wang, Z.-Q.; Jiang, B. Acta Chim. Sinica 1988, 46, 1150 (in Chinese).
|
|
|
(周维善, 王钟麒, 姜标, 化学学报, 1988, 46, 1150.)
|
|
| [13] |
a) Peng, Y.-H.; Wen, Y.-C.; Chen, Y.-Q. Acta Chim. Sinica 1985, 43, 698 (in Chinese).
|
|
(彭逸华, 温业淳, 陈毓群, 化学学报, 1985, 43, 698.)
|
|
|
(b) Wang, Z.-Q.; Jiang, L.-Z.; Zhou, W.-S. Chin. Chem. Lett. 1992, 3, 409.
|
|
|
(c) Jin, C.; Wang, Y.-L.; Sun, B.; Su, W.-K. J. Chem. Res. 2018, 42, 96.
|
|
| [14] |
Nakai, Y.; Moriyama, K.; Togo, H. Eur. J. Org. Chem. 2016, 2016, 768.
|
| [15] |
a) Tan, X. Q.; Song, T.; Wang, Z. T.; Chen, H.; Cui, L.; Li, C. Z. Org. Lett. 2017, 19, 1634.
|
|
(b) Wang, Z. T.; Zhu, L.; Yin, F.; Su, Z. Q.; Li, Z. D.; Li, C. Z. J. Am. Chem. Soc. 2012, 134, 425.
|
|
| [16] |
Camps, P.; Lukach, A. E.; Pujol, X.; Vazquez, S. Tetrahedron 2000, 56, 2703.
|
| [17] |
Candish, L.; Standley, E. A.; Gomez-Suarez, A.; Mukherjee, S.; Glorius, F.Chem.-Eur. J. 2016, 22, 9971.
|
| [18] |
Partridge, J. J.; Faber, S.; Uskokovic, M. R. Helv. Chim. Acta 1974, 57, 84.
|
| [19] |
Xiao, J.; Song, J.-Y.; Lin, B.; Li, W.; Yang, Y.-Q.; Liu, J.-Y.; Hou, Y.; Chen, G.; Li, N. J. Nat. Prod. 2020, 83, 864.
|
| [20] |
Liu, Y.-C.; Feng, N.; Li, W.-W.; Tu, P.-F.; Chen, J.-P.; Han, J.-Y.; Zeng, K.-W. Molecules 2020, 25, 2840.
|
| [21] |
Eguchi, T.; Sai, H.; Takatsuto, S.; Hara, N.; Ikekawa, N. Chem. Pharm. Bull. 1988, 36, 2303.
|
| [1] | 杨帅, 吴杰, 汪亮亮. 抗生素Elansolid A简化衍生物的不对称全合成[J]. 有机化学, 2024, 44(7): 2350-2362. |
| [2] | 李令东, 张维伦, 刘鹏飞, 周子杰, 周豪, 杜中田. 双子季铵盐氯胺的合成及抗菌应用[J]. 有机化学, 2024, 44(6): 2041-2048. |
| [3] | 胡健灵, 张超, 朱文达, 何业谱, 彭姝羚, 陈振强, 李明月, 刘志军, 陈河如. 新型1,2-二取代肼的设计合成及抗癌活性初步研究[J]. 有机化学, 2024, 44(6): 1870-1883. |
| [4] | 梁蕾蕾, 姚家灿, 丁凡, 徐畅, 刘丹丹. 水鬼蕉碱Pancratistatin的合成方法研究进展[J]. 有机化学, 2024, 44(6): 1793-1810. |
| [5] | 秦丽清, 林桂汕, 段文贵, 崔玉成, 杨卯芳, 李芳耀, 李典鹏. 新型长叶烯基萘满并N-酰基吡唑化合物的合成、抗增殖活性、三维定量构效关系及分子对接研究[J]. 有机化学, 2024, 44(6): 1967-1977. |
| [6] | 孙一平, 陈德茂, 何玲, 王祖利. Na2S2O8介导的咪唑并[1,2-α]吡啶与杂芳胺在无金属条件下的C—H胺化反应[J]. 有机化学, 2024, 44(5): 1667-1674. |
| [7] | 刘吉永, 吴明慧, 相君成, 庞怀林, 李斌, 吕亮. 新型含(卤代)烷氧基类双酰胺化合物的合成、杀虫活性及构效关系研究[J]. 有机化学, 2024, 44(5): 1584-1591. |
| [8] | 许芹芳, 胡健灵, 刘园林, 张超, 李明月, 彭姝羚, 刘志军, 陈河如. 具更佳成药性的新型三氮烯化合物的设计、合成及抗癌活性研究[J]. 有机化学, 2024, 44(5): 1606-1619. |
| [9] | 张文生, 郑伟, 左国强, 马科友, 肖合全, 刘改云. 一锅法还原胺化/N-酰基化/Aza-Wittig反应合成3,4-二氢喹唑啉[J]. 有机化学, 2024, 44(5): 1686-1690. |
| [10] | 张雅芳, 黄轩, 林琪, 钟海琼, 翁志强, 吴伟. 噻唑类化合物的合成研究进展[J]. 有机化学, 2024, 44(5): 1458-1479. |
| [11] | 吴游金, 金正盛, 刘咏嘉, 黄文倩, 赵桂龙. 基于NYX-2925 L-脯氨酸衍生的N-甲基-D-天冬氨酸(NMDA)受体部分激动剂的设计、合成和活性研究[J]. 有机化学, 2024, 44(4): 1247-1263. |
| [12] | 张晓, 胡密霞, 杜艳青, 梁凤英, 张笑迎, 额尔敦. 阴离子-π相互作用研究进展[J]. 有机化学, 2024, 44(4): 1181-1196. |
| [13] | 彭天凤, 赵玉祥, 浦绍健, 罗娟, 刘腾, 缪应纯, 沈先福. 过渡金属催化的关键反应在异戊烯基吲哚生物碱全合成中的研究进展[J]. 有机化学, 2024, 44(4): 1160-1180. |
| [14] | 叶浩, 张海滨, 吴亚男, 吴新星. 氮杂吲哚啉及其衍生物的合成研究进展[J]. 有机化学, 2024, 44(4): 1106-1123. |
| [15] | 黄克金, 蔡金博, 王瑞革, 张永红, 王斌, 夏昱, 金伟伟, 李新勇, 刘晨江. 硼促进甘氨酸衍生物的电化学C(sp2)—H溴化反应[J]. 有机化学, 2024, 44(3): 989-996. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||