

镍催化1,3-二烯的不对称氢官能团化反应研究进展
收稿日期: 2024-03-15
修回日期: 2024-06-05
网络出版日期: 2024-06-24
基金资助
国家自然科学基金(22377097); 湖北省自然科学基金(2021CFB556); 湖北省教育厅科学研究计划(Q20221513); 磷资源开发利用教育部工程研究中心开放基金(LCX202305)
Recent Advances in Nickel-Catalyzed Asymmetric Hydrofunctionalization of 1,3-Dienes
Received date: 2024-03-15
Revised date: 2024-06-05
Online published: 2024-06-24
Supported by
National Natural Science Foundation of China(22377097); Natural Science Foundation of Hubei Province of China(2021CFB556); Science Research Foundation of Hubei Provincial Department of Education(Q20221513); Open Fund of Engineering Research Center of Phosphorus Resources Development and Utilization of Ministry of Education(LCX202305)
龙姣 , 刘白雪 , 张双双 , 朱园园 , 古双喜 . 镍催化1,3-二烯的不对称氢官能团化反应研究进展[J]. 有机化学, 2024 , 44(11) : 3309 -3320 . DOI: 10.6023/cjoc202403021
The regioselective asymmetric hydrofunctionalization of 1,3-dienes is a step- and atom-economic method for the synthesis of chiral allylic or homoallylic compounds, which has important application value in the synthesis of natural products and bioactive molecules. Over the years, earth-abundant transition metals have attracted extensive attention in the field of organic synthesis due to their excellent characteristics, such as affordability, accessibility, and environmental friendliness. In this review, the recent progress and status of asymmetric hydrofunctionalization of 1,3-dienes with different nucleophiles via nickel catalysis is summarized. The substrate scope, limitations and mechanism characteristics of related reactions are discussed. According to the different kinds of nucleophiles, it mainly includes asymmetric hydrocarbonation, asymmetric hydroamina- tion, asymmetric hydrophosphinylation and asymmetric hydroalkoxylation.
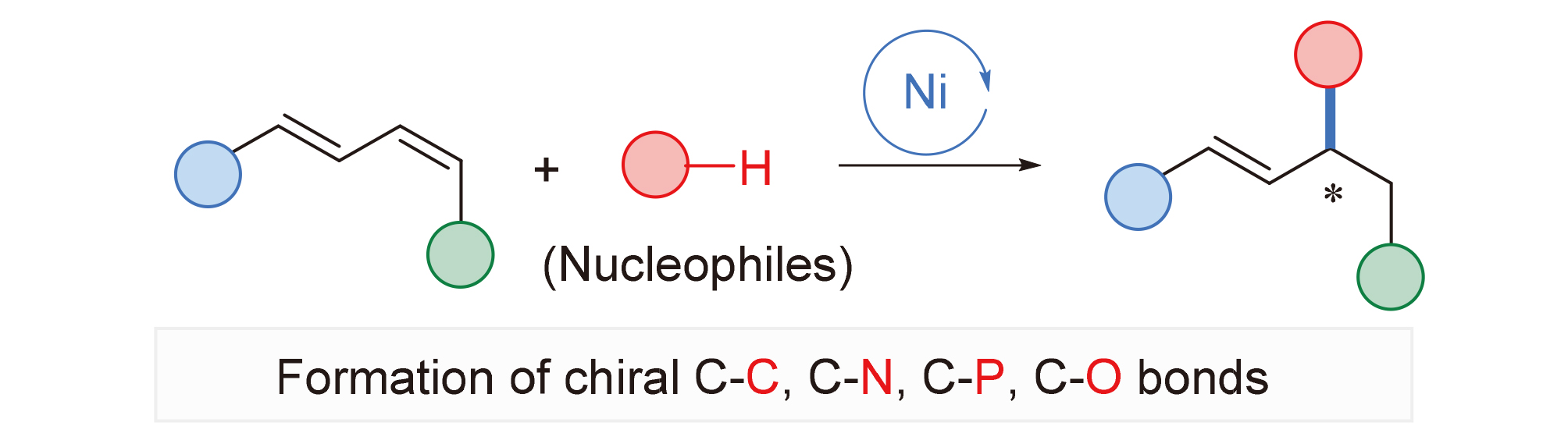
Key words: hydrofunctionalization; 1,3-dienes; asymmetric catalysis; nickel catalyst
| [1] | (a) Newhouse T.; Baran P. S.; Hoffmann R. W. Chem. Soc. Rev. 2009, 38, 3010. |
| [1] | (b) Noyori R. Nat. Chem. 2009, 1, 5. |
| [2] | For selected reviews on hydrofunctionalization of alkenes, see: (a) Crossley S. W. M.; Obradors C.; Martinez R. M.; Shenvi R. A. Chem. Rev. 2016, 116, 8912. |
| [2] | (b) Xiao L.-J.; Ye M.-C.; Zhou Q.-L. Synlett 2018, 30, 361. |
| [2] | (c) Fernández D. F.; Mascare?as J. L.; López F. Chem. Soc. Rev. 2020, 49, 7378. |
| [2] | (d) Liu R. Y.; Buchwald S. L. Acc. Chem. Res. 2020, 53, 1229. |
| [2] | (e) Zhang W.; Wang Z.; Bai X.; Li B. Chin. J. Org. Chem. 2020, 40, 1087 (in Chinese). |
| [2] | (张雯雯, 王紫璇, 白小燕, 李必杰, 有机化学, 2020, 40, 1087.) |
| [2] | (f) Biswas S.; Parsutkar M. M.; Jing S. M.; Pagar V. V.; Herbort J. H.; RajanBabu T. V. Acc. Chem. Res. 2021, 54, 4545. |
| [2] | (g) Zhu S.; Tao R.; He Y. Synlett 2021, 33, 224. |
| [2] | (h) He Y.; Chen J.; Jiang X.; Zhu S. Chin. J. Chem. 2022, 40, 651. |
| [2] | (i) Zhang Z.; Bera S.; Fan C.; Hu X. J. Am. Chem. Soc. 2022, 144, 7015. |
| [3] | For selected reviews on hydrofunctionalization of alkynes, see: (a) Lalic G.; Suess A. Synlett 2016, 27, 1165. |
| [3] | (b) Zheng Y.; Zi W. Tetrahedron Lett. 2018, 59, 2205. |
| [3] | (c) Guo J.; Cheng Z.; Chen J.; Chen X.; Lu Z. Acc. Chem. Res. 2021, 54, 2701. |
| [3] | (d) Nu?ez Bahena E.; Schafer L. L. ACS Catal. 2022, 12, 14934. |
| [3] | (e) Torres-Calis A.; García J. J. ACS Omega 2022, 7, 37008. |
| [4] | For a selected review on hydrofunctionalization of allenes, see: Blieck R.; Taillefer M.; Monnier F. Chem. Rev. 2020, 120, 13545. |
| [5] | Perry G. J. P.; Jia T.; Procter D. J. ACS Catal. 2019, 10, 1485. |
| [6] | Adamson N. J.; Malcolmson S. J. ACS Catal. 2020, 10, 1060. |
| [7] | Flaget A.; Zhang C.; Mazet C. ACS Catal. 2022, 12, 15638. |
| [8] | Li G.; Huo X.; Jiang X.; Zhang W. Chem. Soc. Rev. 2020, 49, 2060. |
| [9] | Cheng Z.; Guo J.; Lu Z. Chem. Commun. 2020, 56, 2229. |
| [10] | For representative works on Rh-catalyzed asymmetric hydrofunctionalization of 1,3-dienes, see: (a) Roberts C. C.; Matías D. M.; Goldfogel M. J.; Meek S. J. J. Am. Chem. Soc. 2015, 137, 6488. |
| [10] | (b) Goldfogel M. J.; Meek S. J. Chem. Sci. 2016, 7, 4079. |
| [10] | (c) Marcum J. S.; Roberts C. C.; Manan R. S.; Cervarich T. N.; Meek S. J. J. Am. Chem. Soc. 2017, 139, 15580. |
| [10] | (d) Yang X.-H.; Dong V. M. J. Am. Chem. Soc. 2017, 139, 1774. |
| [10] | (e) Yang X.-H.; Davison R. T.; Dong V. M. J. Am. Chem. Soc. 2018, 140, 10443. |
| [10] | (f) Yang X.-H.; Davison R. T.; Nie S.-Z.; Cruz F. A.; McGinnis T. M.; Dong V. M. J. Am. Chem. Soc. 2019, 141, 3006. |
| [11] | For representative works on Pd-catalyzed asymmetric hydrofunctionalization of 1,3-dienes, see: (a) O. L?ber; M. Kawatsura; Hartwig J. F. J. Am. Chem. Soc. 2001, 123, 4366. |
| [11] | (b) Adamson N. J.; Hull E.; Malcolmson S. J. J. Am. Chem. Soc. 2017, 139, 7180. |
| [11] | (c) Adamson N. J.; Wilbur K. C. E.; Malcolmson S. J. J. Am. Chem. Soc. 2018, 140, 2761. |
| [11] | (d) Park S.; Malcolmson S. J. ACS Catal. 2018, 8, 8468. |
| [11] | (e) Park S.; Adamson N. J.; Malcolmson S. J. Chem. Sci. 2019, 10, 5176. |
| [12] | (a) Tasker S. Z.; Standley E. A.; Jamison T. F. Nature 2014, 509, 299. |
| [12] | (b) Ananikov V. P. ACS Catal. 2015, 5, 1964. |
| [13] | (a) Clement N. D.; Routaboul L.; Grotevendt A.; Jackstell R.; Beller M. Chem. Eur. J. 2008, 14, 7408. |
| [13] | (b) Behr A.; Becker M.; Beckmann T.; Johnen L.; Leschinski J.; Reyer S. Angew. Chem., Int. Ed. 2009, 48, 3598. |
| [14] | Wang Y.; Liu J.; He Z. Chin. J. Org. Chem. 2023, 43, 2614 (in Chinese). |
| [14] | (王玉超, 刘晋彪, 何智涛, 有机化学, 2023, 43, 2614.) |
| [15] | Sato Y.; Saito N.; Mori M. J. Am. Chem. Soc. 2000, 122, 2371. |
| [16] | Yang Y.; Zhu S.-F.; Duan H.-F.; Zhou C.-Y.; Wang L.-X.; Zhou Q.-L. J. Am. Chem. Soc. 2007, 129, 2248. |
| [17] | Cheng L.; Li M.-M.; Xiao L.-J.; Xie J.-H.; Zhou Q.-L. J. Am. Chem. Soc. 2018, 140, 11627. |
| [18] | (a) Xiao L.-J.; Fu X.-N.; Zhou M.-J.; Xie J.-H.; Wang L.-X.; Xu X.-F.; Zhou Q.-L. J. Am. Chem. Soc. 2016, 138, 2957. |
| [18] | (b) Xiao L. J.; Cheng L.; Feng W. M.; Li M. L.; Xie J. H.; Zhou Q. L. Angew. Chem., Int. Ed. 2017, 57, 461. |
| [19] | Shao W.; Besnard C.; Guénée L.; Mazet C. J. Am. Chem. Soc. 2020, 142, 16486. |
| [20] | Liao L.; Zhang Y.; Wu Z.-W.; Ye Z.-T.; Zhang X.-X.; Chen G.; Yu J.-S. Chem. Sci. 2022, 13, 12519. |
| [21] | Bogdanovic B.; Henc B.; Meister B.; Pauling H.; Wilke G. A. Angew. Chem., Int. Ed. 1972, 11, 1023. |
| [22] | Siv C.; Peiffer G.; Triantaphylides C.; Denis P.; Mortreux A.; Petit F.; Buono G. J. Org. Chem. 1985, 50, 1781. |
| [23] | Zhang A.; RajanBabu T. V. J. Am. Chem. Soc. 2006, 128, 54. |
| [24] | (a) Saha B.; Smith C. R.; RajanBabu T. V. J. Am. Chem. Soc. 2008, 130, 9000. |
| [24] | (b) Shingate B. B.; Hazra B. G. Chem. Rev. 2014, 114, 6349. |
| [25] | Cheng L.; Li M.-M.; Li M.-L.; Xiao L.-J.; Xie J.-H.; Zhou Q.-L. CCS Chem. 2022, 4, 2612. |
| [26] | Li J.-F.; Pan D.; Wang H.-R.; Zhang T.; Li Y.; Huang G.; Ye M. J. Am. Chem. Soc. 2022, 144, 18810. |
| [27] | Saha B.; RajanBabu T. V. Org. Lett. 2006, 8, 4657. |
| [28] | Yu R.; Xing Y.; Fang X. Org. Lett. 2021, 23, 930. |
| [29] | Shirakura M.; Suginome M. Angew. Chem., Int. Ed. 2010, 49, 3827. |
| [30] | Baker R.; Halliday D. E.; Smith T. N. J. Chem. Soc. D 1971, 23, 1583. |
| [31] | Pawlas J.; Nakao Y.; Kawatsura M.; Hartwig J. F. J. Am. Chem. Soc. 2002, 124, 3669. |
| [32] | Tran G.; Shao W.; Mazet C. J. Am. Chem. Soc. 2019, 141, 14814. |
| [33] | Long J.; Wang P.; Wang W.; Li Y.; Yin G. iScience 2019, 22, 369. |
| [34] | For selected reviews on hydrophosphinylation, see: (a) Xu Q.; Han L-B. J. Organomet. Chem. 2011, 696, 130. |
| [34] | (b) Zhao D.; Wang R. Chem. Soc. Rev. 2012, 41, 2095. |
| [34] | (c) Pullarkat S. Synthesis 2015, 48, 493. |
| [34] | (d) Trofimov B.; Gusarova N.; Chernysheva N. Synthesis 2017, 49, 4783. |
| [35] | For representative works on transition metal-catalyzed hydrophosphinylation, see: (a) Nie S.-Z.; Davison R. T.; Dong V. M. J. Am. Chem. Soc. 2018, 140, 16450. |
| [35] | (b) Lu Z.-W.; Zhang H.; Yang Z.; Ding N.; Meng L.; Wang J. ACS Catal. 2019, 9, 1457. |
| [35] | (c) Dai Q.; Liu L.; Qian Y.; Li W.; Zhang J. Angew. Chem., Int. Ed. 2020, 59, 20645. |
| [35] | (d) Yang Z.; Gu X.; Han L.-B.; Wang J. Chem. Sci. 2020, 11, 7451. |
| [35] | (e) Duan S.; Pan A.; Du Y.; Zhu G.; Tian X.; Zhang H.; Walsh P. J.; Yang X. ACS Catal. 2023, 13, 10887. |
| [36] | Long J.; Li Y.; Zhao W.; Yin G. Chem. Sci. 2022, 13, 1390. |
| [37] | (a) González-Belman O.; Brotons-Rufes A.; Tomasini M.; Falivene L.; Caporaso L.; Jiménez-Halla J.; Poater A. Catalysts 2021, 11, 704. |
| [37] | (b) Nanda S. K.; Mallik R. Chem. Eur. J. 2021, 27, 15571. |
| [38] | Kennemur J. L.; Maji R.; Scharf M. J.; List B. Chem. Rev. 2021, 121, 14649. |
| [39] | Tran G.; Mazet C. Org. Lett. 2019, 21, 9124. |
| [40] | Mifleur A.; Suisse I.; Mortreux A.; Sauthier M. Catal. Lett. 2021, 151, 27. |
| [41] | Li Q.; Wang Z.; Dong V. M.; Yang X.-H. J. Am. Chem. Soc. 2023, 145, 3909. |
| [42] | (a) Liu C.; Xie J.; Wu W.; Wang M.; Chen W.; Idres S. B.; Rong J.; Deng L.-W.; Khan S. A.; Wu J. Nat. Chem. 2021, 13, 451. |
| [42] | (b) Feng K.; Chen J.; Gu S.; Wang H.; Chen F. Chin. J. Org. Chem. 2024, 44, 378 (in Chinese). |
| [42] | (冯康博, 陈炯, 古双喜, 王海峰, 陈芬儿, 有机化学, 2024, 44, 378.) |
| [42] | (c) Liu D.; Zhu Y.; Gu S.; Chen F. Chin. J. Org. Chem. 2021, 41, 1002 (in Chinese). |
| [42] | (刘玎, 朱园园, 古双喜, 陈芬儿, 有机化学, 2021, 41, 1002.) |
/
| 〈 |
|
〉 |