有机化学 ›› 2024, Vol. 44 ›› Issue (11): 3309-3320.DOI: 10.6023/cjoc202403021 上一篇 下一篇
综述与进展
龙姣a,c,*( ), 刘白雪a,c, 张双双a,c, 朱园园b, 古双喜a,c,*(
), 刘白雪a,c, 张双双a,c, 朱园园b, 古双喜a,c,*( )
)
收稿日期:2024-03-15
修回日期:2024-06-05
发布日期:2024-06-24
基金资助:
Jiao Longa,c,*( ), Baixue Liua,c, Shuangshuang Zhanga,c, Yuanyuan Zhub, Shuangxi Gua,c,*(
), Baixue Liua,c, Shuangshuang Zhanga,c, Yuanyuan Zhub, Shuangxi Gua,c,*( )
)
Received:2024-03-15
Revised:2024-06-05
Published:2024-06-24
Contact:
*E-mail:Supported by:文章分享
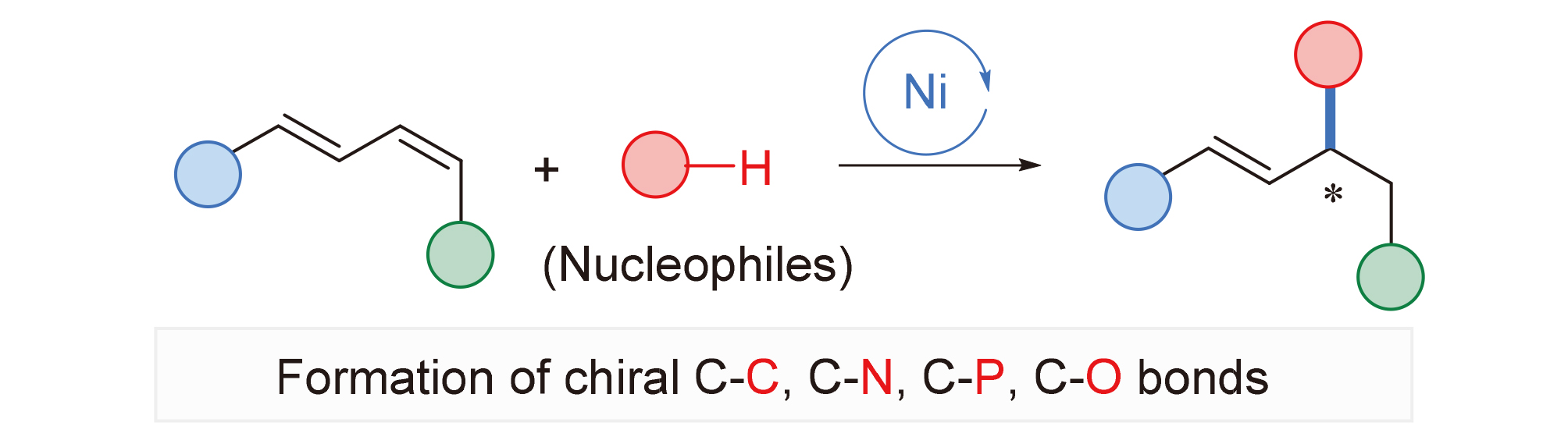
1,3-二烯类化合物的区域选择性不对称氢官能团化反应是一种步骤及原子经济性地合成手性烯丙基或者高烯丙基化合物的方法, 在天然产物和生物活性分子的合成中有着重要的应用价值. 近年来, 丰产金属因其廉价易得、环境友好等优良特性在有机合成领域引起了广泛的关注. 本综述总结了近年来丰产金属镍催化的1,3-二烯与亲核试剂的不对称氢官能团化反应研究进展和现状, 对相关反应的底物范围、局限性以及反应机理进行了概述. 根据亲核试剂种类的不同, 主要包括不对称氢碳化反应、不对称氢胺化反应、不对称氢膦化反应以及不对称氢烷氧基化反应等.
龙姣, 刘白雪, 张双双, 朱园园, 古双喜. 镍催化1,3-二烯的不对称氢官能团化反应研究进展[J]. 有机化学, 2024, 44(11): 3309-3320.
Jiao Long, Baixue Liu, Shuangshuang Zhang, Yuanyuan Zhu, Shuangxi Gu. Recent Advances in Nickel-Catalyzed Asymmetric Hydrofunctionalization of 1,3-Dienes[J]. Chinese Journal of Organic Chemistry, 2024, 44(11): 3309-3320.
| [1] |
(a) Newhouse T.; Baran P. S.; Hoffmann R. W. Chem. Soc. Rev. 2009, 38, 3010.
doi: 10.1039/b821200g pmid: 21378778 |
|
(b) Noyori R. Nat. Chem. 2009, 1, 5.
doi: 10.1038/nchem.143 pmid: 21378778 |
|
| [2] |
For selected reviews on hydrofunctionalization of alkenes, see: (a) Crossley S. W. M.; Obradors C.; Martinez R. M.; Shenvi R. A. Chem. Rev. 2016, 116, 8912.
doi: 10.1021/acs.chemrev.6b00334 pmid: 27461578 |
|
(b) Xiao L.-J.; Ye M.-C.; Zhou Q.-L. Synlett 2018, 30, 361.
pmid: 27461578 |
|
|
(c) Fernández D. F.; Mascareñas J. L.; López F. Chem. Soc. Rev. 2020, 49, 7378.
pmid: 27461578 |
|
|
(d) Liu R. Y.; Buchwald S. L. Acc. Chem. Res. 2020, 53, 1229.
pmid: 27461578 |
|
|
(e) Zhang W.; Wang Z.; Bai X.; Li B. Chin. J. Org. Chem. 2020, 40, 1087 (in Chinese).
pmid: 27461578 |
|
|
(张雯雯, 王紫璇, 白小燕, 李必杰, 有机化学, 2020, 40, 1087.)
doi: 10.6023/cjoc202002017 pmid: 27461578 |
|
|
(f) Biswas S.; Parsutkar M. M.; Jing S. M.; Pagar V. V.; Herbort J. H.; RajanBabu T. V. Acc. Chem. Res. 2021, 54, 4545.
pmid: 27461578 |
|
|
(g) Zhu S.; Tao R.; He Y. Synlett 2021, 33, 224.
pmid: 27461578 |
|
|
(h) He Y.; Chen J.; Jiang X.; Zhu S. Chin. J. Chem. 2022, 40, 651.
pmid: 27461578 |
|
|
(i) Zhang Z.; Bera S.; Fan C.; Hu X. J. Am. Chem. Soc. 2022, 144, 7015.
pmid: 27461578 |
|
| [3] |
For selected reviews on hydrofunctionalization of alkynes, see: (a) Lalic G.; Suess A. Synlett 2016, 27, 1165.
pmid: 36312376 |
|
(b) Zheng Y.; Zi W. Tetrahedron Lett. 2018, 59, 2205.
pmid: 36312376 |
|
|
(c) Guo J.; Cheng Z.; Chen J.; Chen X.; Lu Z. Acc. Chem. Res. 2021, 54, 2701.
pmid: 36312376 |
|
|
(d) Nuñez Bahena E.; Schafer L. L. ACS Catal. 2022, 12, 14934.
pmid: 36312376 |
|
|
(e) Torres-Calis A.; García J. J. ACS Omega 2022, 7, 37008.
doi: 10.1021/acsomega.2c05109 pmid: 36312376 |
|
| [4] |
For a selected review on hydrofunctionalization of allenes, see: Blieck R.; Taillefer M.; Monnier F. Chem. Rev. 2020, 120, 13545.
doi: 10.1021/acs.chemrev.0c00803 pmid: 33301308 |
| [5] |
Perry G. J. P.; Jia T.; Procter D. J. ACS Catal. 2019, 10, 1485.
|
| [6] |
Adamson N. J.; Malcolmson S. J. ACS Catal. 2020, 10, 1060.
|
| [7] |
Flaget A.; Zhang C.; Mazet C. ACS Catal. 2022, 12, 15638.
|
| [8] |
Li G.; Huo X.; Jiang X.; Zhang W. Chem. Soc. Rev. 2020, 49, 2060.
|
| [9] |
Cheng Z.; Guo J.; Lu Z. Chem. Commun. 2020, 56, 2229.
|
| [10] |
For representative works on Rh-catalyzed asymmetric hydrofunctionalization of 1,3-dienes, see: (a) Roberts C. C.; Matías D. M.; Goldfogel M. J.; Meek S. J. J. Am. Chem. Soc. 2015, 137, 6488.
pmid: 30155052 |
|
(b) Goldfogel M. J.; Meek S. J. Chem. Sci. 2016, 7, 4079.
doi: 10.1039/c5sc04908c pmid: 30155052 |
|
|
(c) Marcum J. S.; Roberts C. C.; Manan R. S.; Cervarich T. N.; Meek S. J. J. Am. Chem. Soc. 2017, 139, 15580.
pmid: 30155052 |
|
|
(d) Yang X.-H.; Dong V. M. J. Am. Chem. Soc. 2017, 139, 1774.
pmid: 30155052 |
|
|
(e) Yang X.-H.; Davison R. T.; Dong V. M. J. Am. Chem. Soc. 2018, 140, 10443.
pmid: 30155052 |
|
|
(f) Yang X.-H.; Davison R. T.; Nie S.-Z.; Cruz F. A.; McGinnis T. M.; Dong V. M. J. Am. Chem. Soc. 2019, 141, 3006.
pmid: 30155052 |
|
| [11] |
For representative works on Pd-catalyzed asymmetric hydrofunctionalization of 1,3-dienes, see: (a) O. Löber; M. Kawatsura; Hartwig J. F. J. Am. Chem. Soc. 2001, 123, 4366.
pmid: 29446922 |
|
(b) Adamson N. J.; Hull E.; Malcolmson S. J. J. Am. Chem. Soc. 2017, 139, 7180.
doi: 10.1021/jacs.7b03480 pmid: 29446922 |
|
|
(c) Adamson N. J.; Wilbur K. C. E.; Malcolmson S. J. J. Am. Chem. Soc. 2018, 140, 2761.
doi: 10.1021/jacs.7b13300 pmid: 29446922 |
|
|
(d) Park S.; Malcolmson S. J. ACS Catal. 2018, 8, 8468.
pmid: 29446922 |
|
|
(e) Park S.; Adamson N. J.; Malcolmson S. J. Chem. Sci. 2019, 10, 5176.
pmid: 29446922 |
|
| [12] |
(a) Tasker S. Z.; Standley E. A.; Jamison T. F. Nature 2014, 509, 299.
|
|
(b) Ananikov V. P. ACS Catal. 2015, 5, 1964.
|
|
| [13] |
(a) Clement N. D.; Routaboul L.; Grotevendt A.; Jackstell R.; Beller M. Chem. Eur. J. 2008, 14, 7408.
|
|
(b) Behr A.; Becker M.; Beckmann T.; Johnen L.; Leschinski J.; Reyer S. Angew. Chem., Int. Ed. 2009, 48, 3598.
|
|
| [14] |
Wang Y.; Liu J.; He Z. Chin. J. Org. Chem. 2023, 43, 2614 (in Chinese).
|
|
(王玉超, 刘晋彪, 何智涛, 有机化学, 2023, 43, 2614.)
doi: 10.6023/cjoc202302010 |
|
| [15] |
Sato Y.; Saito N.; Mori M. J. Am. Chem. Soc. 2000, 122, 2371.
|
| [16] |
Yang Y.; Zhu S.-F.; Duan H.-F.; Zhou C.-Y.; Wang L.-X.; Zhou Q.-L. J. Am. Chem. Soc. 2007, 129, 2248.
pmid: 17269780 |
| [17] |
Cheng L.; Li M.-M.; Xiao L.-J.; Xie J.-H.; Zhou Q.-L. J. Am. Chem. Soc. 2018, 140, 11627.
doi: 10.1021/jacs.8b09346 pmid: 30183283 |
| [18] |
(a) Xiao L.-J.; Fu X.-N.; Zhou M.-J.; Xie J.-H.; Wang L.-X.; Xu X.-F.; Zhou Q.-L. J. Am. Chem. Soc. 2016, 138, 2957.
|
|
(b) Xiao L. J.; Cheng L.; Feng W. M.; Li M. L.; Xie J. H.; Zhou Q. L. Angew. Chem., Int. Ed. 2017, 57, 461.
|
|
| [19] |
Shao W.; Besnard C.; Guénée L.; Mazet C. J. Am. Chem. Soc. 2020, 142, 16486.
doi: 10.1021/jacs.0c08319 pmid: 32869987 |
| [20] |
Liao L.; Zhang Y.; Wu Z.-W.; Ye Z.-T.; Zhang X.-X.; Chen G.; Yu J.-S. Chem. Sci. 2022, 13, 12519.
|
| [21] |
Bogdanovic B.; Henc B.; Meister B.; Pauling H.; Wilke G. A. Angew. Chem., Int. Ed. 1972, 11, 1023.
|
| [22] |
Siv C.; Peiffer G.; Triantaphylides C.; Denis P.; Mortreux A.; Petit F.; Buono G. J. Org. Chem. 1985, 50, 1781.
|
| [23] |
Zhang A.; RajanBabu T. V. J. Am. Chem. Soc. 2006, 128, 54.
|
| [24] |
(a) Saha B.; Smith C. R.; RajanBabu T. V. J. Am. Chem. Soc. 2008, 130, 9000.
pmid: 24961597 |
|
(b) Shingate B. B.; Hazra B. G. Chem. Rev. 2014, 114, 6349.
doi: 10.1021/cr4004083 pmid: 24961597 |
|
| [25] |
Cheng L.; Li M.-M.; Li M.-L.; Xiao L.-J.; Xie J.-H.; Zhou Q.-L. CCS Chem. 2022, 4, 2612.
|
| [26] |
Li J.-F.; Pan D.; Wang H.-R.; Zhang T.; Li Y.; Huang G.; Ye M. J. Am. Chem. Soc. 2022, 144, 18810.
|
| [27] |
Saha B.; RajanBabu T. V. Org. Lett. 2006, 8, 4657.
|
| [28] |
Yu R.; Xing Y.; Fang X. Org. Lett. 2021, 23, 930.
|
| [29] |
Shirakura M.; Suginome M. Angew. Chem., Int. Ed. 2010, 49, 3827.
|
| [30] |
Baker R.; Halliday D. E.; Smith T. N. J. Chem. Soc. D 1971, 23, 1583.
|
| [31] |
Pawlas J.; Nakao Y.; Kawatsura M.; Hartwig J. F. J. Am. Chem. Soc. 2002, 124, 3669.
pmid: 11929257 |
| [32] |
Tran G.; Shao W.; Mazet C. J. Am. Chem. Soc. 2019, 141, 14814.
|
| [33] |
Long J.; Wang P.; Wang W.; Li Y.; Yin G. iScience 2019, 22, 369.
|
| [34] |
For selected reviews on hydrophosphinylation, see: (a) Xu Q.; Han L-B. J. Organomet. Chem. 2011, 696, 130.
|
|
(b) Zhao D.; Wang R. Chem. Soc. Rev. 2012, 41, 2095.
|
|
|
(c) Pullarkat S. Synthesis 2015, 48, 493.
|
|
|
(d) Trofimov B.; Gusarova N.; Chernysheva N. Synthesis 2017, 49, 4783.
|
|
| [35] |
For representative works on transition metal-catalyzed hydrophosphinylation, see: (a) Nie S.-Z.; Davison R. T.; Dong V. M. J. Am. Chem. Soc. 2018, 140, 16450.
|
|
(b) Lu Z.-W.; Zhang H.; Yang Z.; Ding N.; Meng L.; Wang J. ACS Catal. 2019, 9, 1457.
|
|
|
(c) Dai Q.; Liu L.; Qian Y.; Li W.; Zhang J. Angew. Chem., Int. Ed. 2020, 59, 20645.
|
|
|
(d) Yang Z.; Gu X.; Han L.-B.; Wang J. Chem. Sci. 2020, 11, 7451.
|
|
|
(e) Duan S.; Pan A.; Du Y.; Zhu G.; Tian X.; Zhang H.; Walsh P. J.; Yang X. ACS Catal. 2023, 13, 10887.
|
|
| [36] |
Long J.; Li Y.; Zhao W.; Yin G. Chem. Sci. 2022, 13, 1390.
|
| [37] |
(a) González-Belman O.; Brotons-Rufes A.; Tomasini M.; Falivene L.; Caporaso L.; Jiménez-Halla J.; Poater A. Catalysts 2021, 11, 704.
|
|
(b) Nanda S. K.; Mallik R. Chem. Eur. J. 2021, 27, 15571.
|
|
| [38] |
Kennemur J. L.; Maji R.; Scharf M. J.; List B. Chem. Rev. 2021, 121, 14649.
|
| [39] |
Tran G.; Mazet C. Org. Lett. 2019, 21, 9124.
|
| [40] |
Mifleur A.; Suisse I.; Mortreux A.; Sauthier M. Catal. Lett. 2021, 151, 27.
|
| [41] |
Li Q.; Wang Z.; Dong V. M.; Yang X.-H. J. Am. Chem. Soc. 2023, 145, 3909.
|
| [42] |
(a) Liu C.; Xie J.; Wu W.; Wang M.; Chen W.; Idres S. B.; Rong J.; Deng L.-W.; Khan S. A.; Wu J. Nat. Chem. 2021, 13, 451.
|
|
(b) Feng K.; Chen J.; Gu S.; Wang H.; Chen F. Chin. J. Org. Chem. 2024, 44, 378 (in Chinese).
|
|
|
(冯康博, 陈炯, 古双喜, 王海峰, 陈芬儿, 有机化学, 2024, 44, 378.)
doi: 10.6023/cjoc202307005 |
|
|
(c) Liu D.; Zhu Y.; Gu S.; Chen F. Chin. J. Org. Chem. 2021, 41, 1002 (in Chinese).
|
|
|
(刘玎, 朱园园, 古双喜, 陈芬儿, 有机化学, 2021, 41, 1002.)
doi: 10.6023/cjoc202007051 |
| [1] | 王家晟, 王泽树, 何卫民, 叶龙武. 邻炔基苯胺氢胺化合成轴手性吲哚研究进展[J]. 有机化学, 2024, 44(6): 1786-1792. |
| [2] | 刘晨光. 含氮芳香性杂环化合物的不对称氢化反应研究进展[J]. 有机化学, 2024, 44(5): 1403-1422. |
| [3] | 高淳, 刘欣, 王明慧, 刘淑贤, 朱婷婷, 张怡康, 郝二军, 杨启亮. 电化学不对称合成反应的研究进展[J]. 有机化学, 2024, 44(3): 673-727. |
| [4] | 杨爽, 房新强. 氮杂环卡宾催化实现的动力学拆分近期研究进展[J]. 有机化学, 2024, 44(2): 448-480. |
| [5] | 陈宛婷, 钟雄威, 邢佳乐, 吴昌书, 高杨. C—N轴手性化合物的不对称催化合成研究进展[J]. 有机化学, 2024, 44(2): 349-377. |
| [6] | 李乾坤, 穆红亮, 简忠保. 中性镍催化制备聚烯烃弹性体: 位阻与次级配位协同作用[J]. 有机化学, 2024, 44(11): 3399-3408. |
| [7] | 姜权彬. 经由氮杂邻联烯醌中间体合成轴手性化合物的研究进展[J]. 有机化学, 2024, 44(1): 159-172. |
| [8] | 程春霞, 吴露平, 沙风, 伍新燕. 手性叔膦-酰胺不对称催化香豆素与Morita-Baylis-Hillman碳酸酯之间的插烯烯丙基烷基化反应[J]. 有机化学, 2023, 43(9): 3188-3195. |
| [9] | 王玉超, 刘晋彪, 何智涛. 钯催化共轭二烯的不对称氢官能团化[J]. 有机化学, 2023, 43(8): 2614-2627. |
| [10] | 罗诚, 尹艳丽, 江智勇. P-手性膦氧化物的不对称合成研究进展[J]. 有机化学, 2023, 43(6): 1963-1976. |
| [11] | 王海清, 杨爽, 张宇辰, 石枫. 邻羟基苄醇参与的催化不对称反应研究进展[J]. 有机化学, 2023, 43(3): 974-999. |
| [12] | 李落墨, 杨小会. 离子转移反应的研究进展[J]. 有机化学, 2023, 43(3): 1036-1044. |
| [13] | 曹伟地, 刘小华. 不对称催化质子化构建α-叔碳羰基化合物研究进展[J]. 有机化学, 2023, 43(3): 961-973. |
| [14] | 方思强, 刘赞娇, 王天利. Atherton-Todd反应的研究进展[J]. 有机化学, 2023, 43(3): 1069-1083. |
| [15] | 吴利城, 伍贤青, 曲景平, 陈宜峰. Quinim配体的探索及其在镍催化烯烃的不对称胺甲酰基-烷基化反应的应用[J]. 有机化学, 2023, 43(12): 4239-4250. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||