

钯催化SO2插入的烯丙基酯与芳基碘化物的还原偶联反应
收稿日期: 2024-04-13
修回日期: 2024-05-13
网络出版日期: 2024-07-15
基金资助
国家自然科学基金(22101259); 浙江省自然科学基金(LQ22B020002)
Pd-Catalyzed Reductive Cross-Coupling of Allylic Esters with Aryl Iodides Involving the Insertion of SO2
Received date: 2024-04-13
Revised date: 2024-05-13
Online published: 2024-07-15
Supported by
National Natural Science Foundation of China(22101259); Zhejiang Provincial Natural Science Foundation(LQ22B020002)
张鑫伟 , 林水侦 , 黄晓雷 . 钯催化SO2插入的烯丙基酯与芳基碘化物的还原偶联反应[J]. 有机化学, 2024 , 44(11) : 3456 -3466 . DOI: 10.6023/cjoc202404019
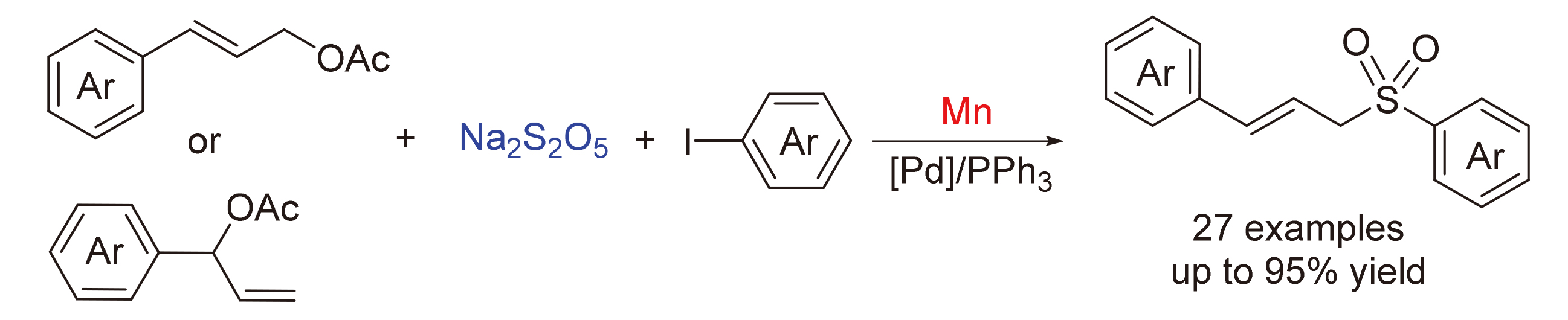
A Pd-catalyzed electrophilic-electrophilic reductive cross-coupling of allylic esters with aryl iodides involving the insertion of SO2 has been reported. This reaction employs manganese powder as reductant, cheap and readily available sodium metabisulfite as SO2 substitute, and features good reactivity, high rselectivity and broad substrate scope.
| [1] | Meadows D. C.; Gervay-Hague J. Med. Res. Rev. 2006, 26, 793. |
| [2] | Li P.; Wang L.; Wang X. J. Heterocycl. Chem. 2021, 58, 28. |
| [3] | (a) Yang B.; Lu Y.; Duan L.; Ma X.; Xia Y.; Huang X. ACS Omega 2023, 8, 10100. |
| [3] | (b) Zhang X.; Lu Y.; Wang H.; Chen M.; Lin S.; Huang X. ACS Omega 2024, 9, 1738. |
| [4] | (a) Engberts J. B. F. N. The Chemistry of Sulphones and Sulphoxides, Eds.: Patai, S.; Rappoport, Z.; Stirling, C., John Wiley and Sons, Chichester, 1988, Vols. 107, 684. |
| [4] | (b) B?ckvall J.-E.; Chinchilla R.; Nájera C.; Yus M. Chem. Rev. 1998, 98, 2291. |
| [5] | Lattanzi A. Comprehensive Organic Synthesis, 2nd ed., Vol. 7, Ed.: Knochel, P., Elsevier, 2014, pp. 837-879. |
| [6] | (a) Guo Y.; Wang G.; Wei L.; Wan J.-P. J. Org. Chem. 2019, 84, 2984. |
| [6] | (b) Zheng X.; Wan J.-P. Adv. Synth. Catal. 2019, 361, 5690. |
| [6] | (c) Dong D.-Q.; Han Q.-Q.; Yang S.-H.; Song J.-C.; Li N.; Wang Z.-L.; Xu X.-M. ChemistrySelect 2020, 5, 13103. |
| [6] | (d) Guo Y.; Liu Y.; Wan J.-P. Chin. Chem. Lett. 2022, 33, 855. |
| [6] | (e) Xu L.; Lü L.; Wang X. Chin. J. Org. Chem. 2023, 43, 3644 (in Chinese). |
| [6] | (许力, 吕兰兰, 王香善, 有机化学, 2023, 43, 3644.) |
| [6] | (f) Liu C.; Li Y.; Zhang Q. Chin. J. Org. Chem. 2023, 43, 1091 (in Chinese). |
| [6] | (刘春阳, 李燕, 张前, 有机化学, 2023, 43, 1091.) |
| [7] | For selected reviews: (a) Qiu G.-S.; Lai L.-F.; Cheng J.; Wu J. Chem. Commun. 2018, 54, 10405. |
| [7] | (b) Qiu G.-S.; Zhou K.-D.; Gao L.; Wu J. Org. Chem. Front. 2018, 5, 691. |
| [7] | (c) Ye S.; Li X.; Xie W.; Wu J. Eur. J. Org. Chem. 2020, 2020, 1274. |
| [7] | (d) Zeng D.; Wang M.; Deng W.-P.; Jiang X.-F. Org. Chem. Front. 2020, 7, 3956. |
| [7] | (e) Joseph D.; Idris M. A.; Chen J.; Lee S. ACS Catal. 2021, 11, 4169. |
| [7] | (f) Wang M.; Jiang X. Chem. Rec. 2021, 21, 3338. |
| [7] | (g) de Souza E. L. S.; Oliveira C. C. Eur. J. Org. Chem. 2023, 26, e202300073. |
| [8] | For selected examples: (a) Johnson M. W.; Bagley S. W.; Mankad N. P.; Bergman R. G.; Mascitti V.; Toste F. D. Angew. Chem., Int. Ed. 2014, 53, 4404. |
| [8] | (b) Shavnya A.; Hesp K. D.; Mascitti V.; Smith A. C. Angew. Chem., Int. Ed. 2015, 54, 13571. |
| [8] | (c) Zheng D.; Chen M.; Yao L.; Wu J. Org. Chem. Front. 2016, 3, 985. |
| [8] | (d) Chen Y.; Willis M. C. Chem. Sci. 2017, 8, 3249. |
| [8] | (e) Zhu H.; Shen Y.; Deng Q.; Chen J.; Tu T. ACS Catal. 2017, 7, 4655. |
| [8] | (f) Zhu H.; Shen Y.; Deng Q.; Chen J.; Tu T. Chem. Commun. 2017, 53, 12473. |
| [8] | (g) Adenot A.; Char J.; von Wolff N.; Lefèvre G.; Anthore- Dalion L.; Cantat T. Chem. Commun. 2019, 55, 12924. |
| [8] | (h) Adenot A.; Anthore-Dalion L.; Nicolas E.; Berthet J.-C.; Thuéry P.; Cantat T. Chem.-Eur. J. 2021, 27, 18047. |
| [9] | (a) Meng Y.; Wang M.; Jiang X. Angew. Chem., Int. Ed. 2020, 59, 1346. |
| [9] | (b) Meng Y.; Wang M.; Jiang X. CCS Chem. 2021, 3, 17. |
| [10] | Xiong B.; Zhang J.; Wang T.; Zhang X.; Cheng G.; Lian Z. Org. Chem. Front. 2023, 10, 3567. |
| [11] | (a) Zhang Q.; Ying Y.; Zhang H.; Xu L.; Lin X.; Huang X. Chin. J. Org. Chem. 2024, 44, 2033 (in Chinese). |
| [11] | (张倩, 应垚璐, 张泓银, 徐林博, 林新奎, 黄晓雷, 有机化学, 2024, 44, 2033.) |
| [11] | (b) Zhang X.; Lu Y.; Zhang S.; Lin S.; Chen M.; Huang X. New J. Chem. 2024, 48, 5101. |
| [12] | Deng W.; Li M.; Fan J.; Cheng X. Nat. Commun. 2022, 13, 5642. |
/
| 〈 |
|
〉 |