

硼促进Co催化使用CO2和H2实现仲芳香胺N-甲基化
收稿日期: 2024-05-31
修回日期: 2024-07-16
网络出版日期: 2024-08-19
基金资助
国家自然科学基金(22022204); 国家自然科学基金(22102197); 江苏省国家自然基金(BK20211096); 江苏省国家自然基金(BK20211093)
Boron-Promoted Co-Catalyzed N-Methylation of Secondary Aromatic Amines with CO2 and H2
Received date: 2024-05-31
Revised date: 2024-07-16
Online published: 2024-08-19
Supported by
National Natural Science Foundation of China(22022204); National Natural Science Foundation of China(22102197); National Science Foundation of Jiangsu Province(BK20211096); National Science Foundation of Jiangsu Province(BK20211093)
石亲 , 李臻 , 何林 , 李玉东 , 李跃辉 . 硼促进Co催化使用CO2和H2实现仲芳香胺N-甲基化[J]. 有机化学, 2024 , 44(10) : 3233 -3240 . DOI: 10.6023/cjoc202405049
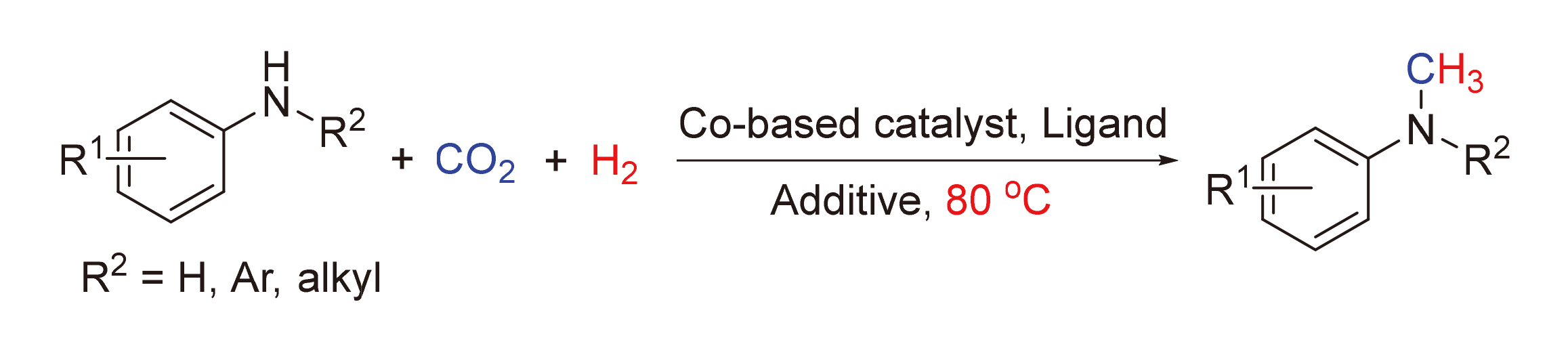
Development of catalytic methods using CO2/H2 as methylating reagent for selective methylation of amines is highly attractive. Herein, the methylation of N—H bond via boron promoted activation of Co-formate intermediates is reported. This catalytic system showed excellent functional group tolerance with high catalytic activity, and a series of methylated products were acquired in moderate to excellent yields under mild conditions (e.g. 80 ℃ or 60 ℃). It was inferred that imine complex C was the crucial intermediate formed via dehydration of species B, providing efficient C—N coupling for the selective N-methylation of secondary aromatic amines with CO2/H2.
Key words: CO2/H2; N-methylation; cobalt catalysis
| [1] | (a) Chatterjee, J.; Rechenmacher, F.; Kessler, H. Angew. Chem., Int. Ed. 2013, 52, 254. |
| [1] | (b) Vitaku, E.; Smith, D. T.; Njardarson, J. T. J. Med. Chem. 2014, 57, 10257. |
| [2] | (a) Malkov, A. V.; Vranková, K.; Cerny, M.; Kocovsky, P. J. Org. Chem. 2009, 74, 8425. |
| [2] | (b) Fatemeh, N. H.; Farasat, Z.; Nabavizadeh, S. M.; Wu, G.; Abu-Omar, M. M. J. Orgnomet. Chem. 2019, 880, 232. |
| [3] | Atkinson, B. N.; Williams, J. M. J. ChemCatChem 2014, 6, 1860. |
| [4] | (a) Auerbach, J.; Zamore, M.; Weinreb, S. M. J. Org. Chem. 1976, 41, 725. |
| [4] | (b) Basha, A.; Orlando, J.; Weinreb, S. M. Synth. Commun. 1977, 7, 549. |
| [5] | (a) Sch?ffner, B.; Sch?ffner, F.; Verevkin, S. P.; B?rner, A. Chem. Rev. 2010, 110, 4554. |
| [5] | (b) Ji, Y.; Sweeney, J.; Zoglio, J.; Gorin, D. J. J. Org. Chem. 2013, 78, 11606. |
| [5] | (c) Fiorani, G.; Perosa, A.; Selva, M. Green Chem. 2018, 20, 288. |
| [6] | Alenazi, N. A.; Lai, E. P. C.; Manthorpe, J. M. J. Mol. Recognit. 2014, 27, 755. |
| [7] | Méndez, M. V.; Heredia, D. A.; Larghi, E. L.; Bracca, A. B. J.; Kaufman, T. S. RSC Adv. 2017, 7, 28298. |
| [8] | Kaithal, A.; H?lscher, M.; Leitner, W. Chem. Sci. 2021, 12, 976. |
| [9] | (a) Garces, L. J.; Makwana, V. D.; Hincapie, B.; Sacco, A.; Suib, S. L. J. Catal. 2003, 217, 107. |
| [9] | (b) Liu, Z.; Yang, Z.; Yu, X.; Zhang, H.; Yu, B.; Zhao, Y.; Liu, Z. Adv. Synth. Catal. 2017, 359, 4278. |
| [9] | (c) Tsarev, V. N.; Morioka, Y.; Caner, J.; Wang, Q.; Ushimaru, R.; Kudo, A.; Naka, H.; Saito, S. Org. Lett. 2015, 17, 2530. |
| [9] | (d) Liang, R.; Li, S.; Wang, R.; Lu, L.; Li, F. Org. Lett. 2017, 19, 5790. |
| [10] | (a) Guo, Z.; Zhang, B.; Wei, X.; Xi, C. ChemSusChem 2018, 11, 2296. |
| [10] | (b) Maji, S.; Das, A.; Mandal, S. K. Chem. Sci. 2021, 12, 12174. |
| [10] | (c) Zou, Q.; Long, G.; Zhao, T.; Hu, X. Green Chem. 2020, 22, 1134. |
| [11] | (a) Artz, J.; Müller, T. E.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Chem. Rev. 2018, 118, 434. |
| [11] | (b) Shindell, D.; Smith, C. J. Nature 2019, 573, 408. |
| [11] | (c) Figueres, C.; Le, Quéré, C.; Mahindra, A.; B?te, O.; Whiteman, G.; Peters, G.; Guan, D. Nature 2018, 564, 27. |
| [12] | (a) Ren, X.; Zheng, Z.; Zhang, L.; Wang, Z.; Xia, C.; Ding, K. Angew. Chem., Int. Ed. 2017, 56, 310. |
| [12] | (b) Hua, K.; Liu, X.; Wei, B.; Shao, Z.; Deng, Y.; Zhong, L.; Wang, H.; Sun, Y. Green Chem. 2021, 23, 8040. |
| [13] | (a) Xia, S.-P.; Ding, G.-R.; Zhang, R.; Han, L.-J.; Xu, B.-H.; Zhang, S.-J. Green Chem. 2021, 23, 3073. |
| [13] | (b) Yang, J.; Liu, J.; Ge, Y.; Huang, W.; Schneider, C.; Dühren, R.; Franke, R.; Neumann, H.; Jackstell, R.; Beller, M. Chem. Commun. 2020, 56, 5235. |
| [13] | (c) Gehrtz, P. H.; Hirschbeck, V.; Fleischer, I. Chem. Commun. 2015, 51, 12574. |
| [13] | (d) Khan, M. U.; Khan, S. U.; Kiriratnikom, J.; Zareen, S.; Zhang, X. Chin. Chem. Lett. 2022, 33, 1081. |
| [14] | (a) Yang, D.-T.; Zhu, M.; Schiffer, Z. J.; Williams, K.; Song, X.; Liu, X.; Manthiram, K. ACS Catal. 2019, 9, 4699. |
| [14] | (b) Jiang, Y.-X.; Liao, L.-L.; Gao, T.-Y.; Xu, W.-H. Zhang, W.; Song, L.; Sun, G.-Q.; Ye, J.-H.; Lan, Y.; Yu, D.-G. Nat. Synth. 2024, 3, 394. |
| [15] | (a) Wang, H.; Li, Y.; Sun, Y.; Li, Y. J. Org. Chem. 2023, 88, 8835. |
| [15] | (b) Wang, H.; Li, C.; Li, Y.; Chen, J.; Liu, S.; Li, Y.; Org. Chem. Front. 2024, 11, 1322. |
| [16] | (a) Huang, Y.; Wang, Y.; Wu, Y.; Yu, Y.; Zhang, B. Sci. China Chem. 2022, 65, 204. |
| [16] | (b) Wang, M.; Chu, L.-Y.; Li, Z.-Y.; Messinis, A. M.; Ding, Y.-Q.; Hu, L.; Ma, J.-B. J. Phys. Chem. Lett. 2021, 12, 3490. |
| [16] | (c) Brahmayya, M.; Dai, S. A.; Suen, S.-Y. J. CO2 Util. 2017, 22, 135. |
| [16] | (d) Du, C.; Chen, Y. Chin. J. Chem. 2020, 38, 1057. |
| [16] | (e) Du, C.; Chen, Y. Acta Chim. Sinica 2020, 78, 938. (in Chinese) |
| [16] | (杜重阳, 陈耀峰, 化学学报, 2020, 78, 938.) |
| [17] | (a) Dong, Y.; Yang, P.; Zhao, S.; Li, Y. Nat. Commun. 2020, 11, 4096. |
| [17] | (b) Wang, H.; Dong, Y.; Zheng, C.; Sandoval, C. A.; Wang, X.; Makha, M.; Li, Y. Chem 2018, 4, 2883. |
| [17] | (c) Wang, H.; Li, Y.; Liu, S.; Makha, M.; Bai, J.-F.; Li, Y. ChemSusChem 2022, 15, e202200227. |
| [17] | (d) Jiang, X.; Huang, Z.; Makha, M.; Du, C.-X.; Zhao, D.; Wang, F.; Li, Y. Green Chem. 2020, 22, 5317. |
| [17] | (e) Huang, Z.; Jiang, X.; Zhou, S.; Yang, P.; Du, C.-X.; Li, Y. ChemSusChem 2019, 12, 3054. |
| [17] | (f) Du, M.; Sun, Y.; Zhao, J.; Hu, H.; Sun, L.; Li, Y. Chin. Chem. Lett. 2023, 34, 108269. |
| [17] | (g) Wang, T.; Yang, J.; Chen, J.; He, Q.; Li, Z.; Lei, L.; Lu, J.; Leung, M. K. H.; Yang, B.; Hou, Y. Chin. Chem. Lett. 2020, 31, 1438. |
| [17] | (h) Liu, Y.; Yang, H.; Fan, X.; Shan, B.; Meyer, T. J. Chin. Chem. Lett. 2022, 33, 4691. |
| [18] | (a) Ma, S.-S.; Sun, R.; Zhang, Z.-H.; Guan, P.-X.; Lin, J.-Q.; Li, C.-S.; Xu, B.-H. Green Chem. 2023, 25, 8625. |
| [18] | (b) Ren, C.; Terazzi, C.; Werner, T. Green Chem. 2023, 26, 439. |
| [18] | (c) Murata, T.; Hiyoshi, M.; Maekawa, S.; Saiki, Y.; Ratanasak, M.; Hasegawa, J.; Ema, T. Green Chem. 2022, 24, 2385. |
| [18] | (d) Zou, Q.; Yi, Y.; Zhao, T.; Liu, F.; Kang, C.; Hu, X. J. CO2 Util. 2021, 50, 101590. |
| [18] | (e) Xia, H.-M.; Zhang, F.-L.; Ye, T.; Wang, Y.-F. Angew. Chem., nt. Ed. 2018, 57, 11770. |
| [18] | (f) Zhang, H.; Zhang, Y.; Gao, K. Org. Chem. Front. 2023, 10, 2491. |
| [19] | Li, Y.; Fang, X.; Junge, K.; Beller, M. Angew. Chem., Int. Ed. 2013, 52, 9568. |
| [20] | Ren, C.; Terazzi, C.; Werner, T. Green Chem. 2024, 26, 439. |
| [21] | Beydoun, K.; vom Stein, T.; Klankermayer, J.; Leitner, W. Angew. Chem., Int. Ed. 2013, 52, 9554. |
| [22] | Li, Y.; Sorribes, I.; Yan, T.; Junge, K.; Beller, M. Angew. Chem., Int. Ed. 2013, 52, 12156. |
| [23] | (a) He, Z.; Liu, H.; Qian, Q.; Lu, L.; Guo, W.; Zhang, L.; Han, B. Sci. China Chem. 2017, 60, 927. |
| [23] | (b) Paul, B.; Panja, D.; Kundu, S. Org. Lett. 2019, 21, 5843. |
| [23] | (c) Dang, T.; Ramalingam, B.; Seayad, A. M. ACS Catal. 2015, 5, 4082. |
| [23] | (d) Huang, M.; Cai, X.; Liu, Y.; Ke, Z. Chin. Chem. Lett. 2024, 35, 109323. |
| [24] | Toyao, T.; Siddiki, S. M. A. H.; Morita, Y.; Kamachi, T.; Touchy, A. S.; Onodera, W.; Kon, K.; Furukawa, S.; Ariga, H.; Asakura, K.; Yoshizawa, K.; Shimizu, K.-I. Chem.-Eur. J. 2017, 23, 14848. |
| [25] | Jo, E.-A.; Lee, J.-H.; Jun, C.-H. Chem. Commun. 2008, 44, 5779. |
| [26] | (a) Wang, L.-M.; Jenkinson, K.; Wheatley, A. E. H.; Kuwata, K.; Saito, S.; Naka, H. ACS Sustainable Chem. Eng. 2018, 6, 15419. |
| [26] | (b) Cui, X.; Zhang, Y.; Deng, Y.; Shi, F. Chem. Commun. 2014, 50, 13521. |
| [26] | (c) Cho, J. H.; Ha, Y.; Cho, A.; Park, J.; Choi, J.; Won, Y.; Kim, H.; Kim, B. M. Catal. Sci. Technol. 2022, 12, 3524. |
| [26] | (d) Saito, Y.; Senzaki, T.; Nishizawa, K.; Kobayashi, S. Green Chem. 2023, 25, 7524. |
| [27] | (a) Toyao, T.; Siddiki, S. M. A. H.; Ishihara, K.; Kon, K.; Onodera, W.; Shimizu, K. Chem. Lett. 2017, 46, 68. |
| [27] | (b) Zhu, L.; Wang, L.-S.; Li, B.; Li, W.; Fu, B. Catal. Sci. Technol. 2016, 6, 6172. |
| [28] | Du, X.-L.; Tang, G.; Bao, H.-L.; Jiang, Z.; Zhong, Z.-H.; Su, D.; Wang, J.-Q. ChemSusChem 2015, 8, 3489. |
| [29] | Ke, Z.; Zhao, Y.; Li, R.; Wang, H.; Zeng, W.; Tang, M.; Han, B.; Liu, Z. Green Chem. 2021, 23, 9147. |
| [30] | Shi, Q.; Hu, H.; Du, M.; Sun, Y.; Li, Y.; Li, Y. Org. Lett. 2023, 25, 7100. |
| [31] | Fu, M.-C.; Shang, R.; Cheng, W.-M.; Fu, Y. Angew. Chem., Int. Ed. 2015, 54, 9042. |
| [32] | Zhang, Q.; Lin, X.-T.; Fukaya, N.; Fujitani, T.; Sato, K.; Choi, J.-C. Green Chem. 2021, 22, 8414. |
| [33] | Li, X.-D.; Xia, S.-M.; Chen, K.-H.; Liu, X.-F.; Li, H.-R.; He, L.-N. Green Chem. 2018, 20, 4853. |
| [34] | Li, J.; Huang, C.; Wen, D.; Zheng, Q.; Tu, B.; Tu, T. Org. Lett. 2021, 23, 687. |
| [35] | Larionova, N. A.; Ondozabal, J. M.; Smith, E. G.; Cambeiro, X. C. Org. Lett. 2021, 23, 5383. |
| [36] | Yamada, S.; Gavryushin, A.; Knochel, P. Angew. Chem., nt. Ed. 2010, 49, 2215. |
| [37] | Xu, Y.; Zhang, Z.; Qiu, C.; Chen, S.; Ling, X.; Su, C. ChemSusChem 2020, 14, 582. |
| [38] | Chen, Z.; Zeng, H.; Gong, H.; Wang, H.; Li, C.-J. Chem. Sci. 2015, 6, 4174. |
| [39] | Jiang, X.; Wang, C.; Wei, Y.; Xue, D.; Liu, Z.; Xiao, J. Chem.-Eur. J. 2014, 20, 58. |
| [40] | Basak, S.; Alvarez-Montoya, A.; Winfrey, L.; Melen, R. L.; Pulis, A. P. ACS. Catal. 2020, 10, 4835. |
| [41] | Matsumoto, K.; Yoshida, M.; Shindo, M. Angew. Chem., Int. Ed. 2016, 55, 5272. |
| [42] | Paquette, L. A.; Kuhla, D. E.; Barrett, J. H. J. Org. Chem. 1969, 34, 2879. |
/
| 〈 |
|
〉 |