

铁催化二氧化碳选择性氢化、硼氢化和硅氢化
Iron-Catalyzed Selective Hydrogenation and Hydroboration/Hydrosilylation of CO2
Received date: 2024-06-28
Revised date: 2024-07-27
Online published: 2024-08-26
赵秋婷 , 王文光 . 铁催化二氧化碳选择性氢化、硼氢化和硅氢化[J]. 有机化学, 2024 , 44(10) : 3106 -3116 . DOI: 10.6023/cjoc202405032
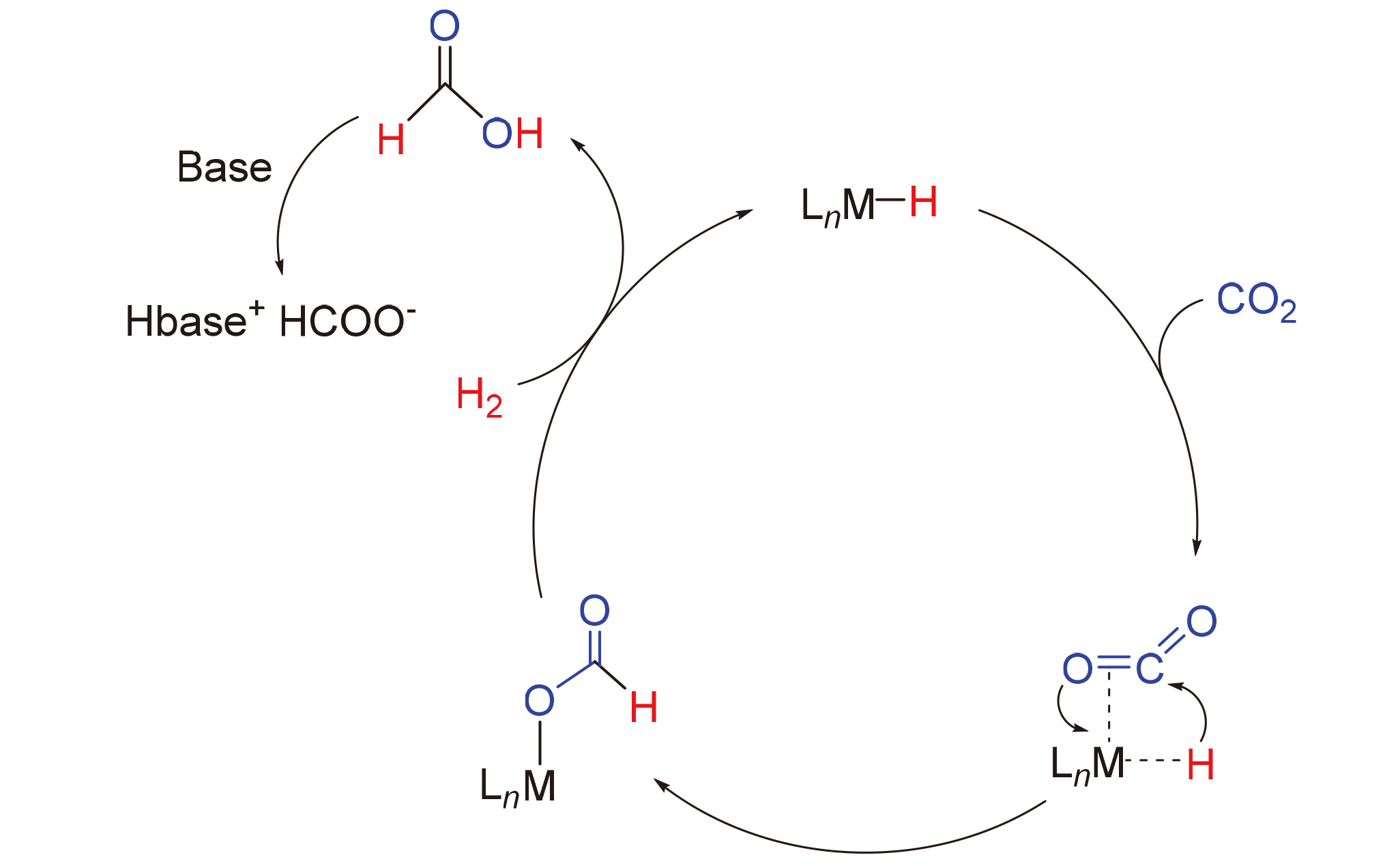
Carbon dioxide (CO2) serves as a sustainable carbon source for building biomass, fossil fuels, and organic chemicals. Converting CO2 into value-added chemicals or fuels is an ideal approach to achieve carbon cycling. The reduction and conversion of CO2, a pivotal aspect of C1 chemistry, have long been a subject of intense research interest. Previous studies have demonstrated that through transition metal catalysis, hydrogen, boranes, and silanes (E—H, E=H, B or Si) act as effective reducing agents to transform CO2 into a range of C1 chemicals, such as formate, formaldehyde, and methanol. Over the past decade, research focus in this field has shifted towards utilizing cost-effective metals as catalysts for selective CO2 reduction. A comprehensive review of homogeneous iron-catalyzed CO2 reduction using E—H is presented, emphasizing reaction mechanisms and selectivity.
| [1] | Keim, W. Pure Appl. Chem. 1986, 58, 825. |
| [2] | Bhaskar, K.; Das, P. C. B.S. Thesis, National Institute of Technology, Rourkela, 2007. |
| [3] | Cooper, A. I. J. Mater. Chem. 2000, 10, 207. |
| [4] | Inoue, Y.; Izumida, H.; Sasaki, Y.; Hashimoto, H. Chem. Lett. 1976, 5, 863. |
| [5] | Behr, A.; Nowakowski, K. Adv. Inorg. Chem. 2014, 66, 223. |
| [6] | Wang, W. H.; Himeda, Y.; Muckerman, J. T.; Manbeck, G. F.; Fujita, E. Chem. Rev. 2015, 115, 12936. |
| [7] | Sordakis, K.; Tang, C.; Vogt, L. K.; Junge, H.; Dyson, P. J.; Beller, M.; Laurenczy, G. Chem. Rev. 2018, 118, 372. |
| [8] | Huang, W.; Qiu, L.; Ren, F.; He, L. Chin. J. Org. Chem. 2021, 41, 3914. (in Chinese) |
| [8] | (黄文斌, 邱丽琪, 任方煜, 何良年, 有机化学, 2021, 41, 3914.) |
| [9] | Zhang, L.; Han, Z.; Zhao, X.; Wang, Z.; Ding, K. Angew. Chem., Int. Ed. 2015, 54, 6186. |
| [10] | Chakraborty, S.; Bhattacharya, P.; Dai, H.; Guan, H. Acc. Chem. Res. 2015, 48, 1995. |
| [11] | Singh, T.; Jalwal, S.; Chakraborty, S. Asian J. Org. Chem. 2022, 11, e202200330. |
| [12] | Das, C.; Grover, J.; Tannu; Das, A.; Maiti, D.; Dutta, A.; Lahiri, G. K. Dalton Trans. 2022, 51, 8160. |
| [13] | Cauwenbergh, R.; Goyal, V.; Maiti, R.; Natte, K.; Das, S. Chem. Soc. Rev. 2022, 51, 9371. |
| [14] | Chakraborty, S.; Zhang, J.; Krause, J. A.; Guan, H. J. Am. Chem. Soc. 2010, 132, 8872. |
| [15] | Federsel, C.; Ziebart, C.; Jackstell, R.; Baumann, W.; Beller, M. Chem. Eur. J. 2012, 18, 72. |
| [16] | Evans, G. O.; Newell, C. J. Inorg. Chim. Acta 1978, 31, L387. |
| [17] | Tai, C.-C.; Chang, T.; Roller, B.; Jessop, P. G. Inorg. Chem. 2003, 42, 7340. |
| [18] | Federsel, C.; Boddien, A.; Jackstell, R.; Jennerjahn, R.; Dyson, P. J.; Scopelliti, R.; Laurenczy, G.; Beller, M. Angew. Chem., Int. Ed. 2010, 49, 9777. |
| [19] | Ziebart, C.; Federsel, C.; Anbarasan, P.; Jackstell, R.; Baumann, W.; Spannenberg, A.; Beller, M. J. Am. Chem. Soc. 2012, 134, 20701. |
| [20] | Fong, H.; Peters, J. C. Inorg. Chem. 2015, 54, 5124. |
| [21] | Montandon-Clerc, M.; Laurenczy, G. J. Catal. 2018, 362, 78. |
| [22] | Langer, R.; Diskin-Posner, Y.; Leitus, G.; Shimon, L. J. W.; Ben-David, Y.; Milstein, D. Angew. Chem., Int. Ed. 2011, 50, 9948. |
| [23] | Rivada-Wheelaghan, O.; Dauth, A.; Leitus, G.; Diskin-Posner, Y.; Milstein, D. Inorg. Chem. 2015, 54, 4526. |
| [24] | Bertini, F.; Gorgas, N.; St?ger, B.; Peruzzini, M.; Veiros, L. F.; Kirchner, K.; Gonsalvi, L. ACS Catal. 2016, 6, 2889. |
| [25] | Zhang, Y.; MacIntosh, A. D.; Wong, J. L.; Bielinski, E. A.; Williard, P. G.; Mercado, B. Q.; Hazari, N.; Bernskoetter, W. H. Chem. Sci. 2015, 6, 4291. |
| [26] | Curley, J. B.; Smith, N. E.; Bernskoetter, W. H.; Hazari, N.; Mercado, B. Q. Organometallics 2018, 37, 3846. |
| [27] | Jayarathne, U.; Hazari, N.; Bernskoetter, W. H. ACS Catal. 2018, 8, 1338. |
| [28] | Zhu, F.; Zhu-Ge, L.; Yang, G.; Zhou, S. ChemSusChem 2015, 8, 609. |
| [29] | Casey, C. P.; Guan, H. J. Am. Chem. Soc. 2009, 131, 2499. |
| [30] | Coleman, M. G.; Brown, A. N.; Bolton, B. A.; Guan, H. Adv. Synth. Catal. 2010, 352, 967. |
| [31] | Berkessel, A.; Reichau, S.; H?h, A. V. D.; Leconte, N.; Meud?rfl, J.-M. Organometallics 2011, 30, 3880. |
| [32] | Fleischer, S.; Zhou, S.; Junge, K.; Beller, M. Angew. Chem., Int. Ed. 2013, 52, 5120. |
| [33] | Fleischer, S.; Zhou, S.; Werkmeister, S.; Junge, K.; Beller, M. Chem.-Eur. J. 2013, 19, 4997. |
| [34] | Kamitani, M.; Nishiguchi, Y.; Tada, R.; Itazaki, M.; Nakazawa, H. Organometallics 2014, 33, 1532. |
| [35] | Lu, X.; Cheng, R.; Turner, N.; Liu, Q.; Zhang, M.; Sun, X. J. Org. Chem. 2014, 79, 9355. |
| [36] | Yu, X.; Pang, M.; Zhang, S.; Hu, X.; Tung, C.-H.; Wang, W. J. Am. Chem. Soc. 2018, 140, 11454. |
| [37] | Gao, H.; Jia, J.; Tung, C.-H.; Wang, W. Organometallics 2023, 42, 944. |
| [38] | Coufourier, S.; Gaillard, S.; Clet, G.; Serre, C.; Daturi, M.; Renaud, J.-L. Chem. Commun. 2019, 55, 4977. |
| [39] | Coufourier, S.; Gaillard, Q. G.; Lohier, J. F.; Poater, A.; Gaillard, S.; Renaud, J.-L. ACS Catal. 2020, 10, 2108. |
| [40] | Kothandaraman, J.; Goeppert, A.; Czaun, M.; Olah, G. A.; Prakash, G. K. S. Green Chem. 2016, 18, 5831. |
| [41] | Kar, S.; Goeppert, A.; Galvan, V.; Chowdhury, R.; Olah, J.; Prakash, G. K. S. J. Am. Chem. Soc. 2018, 140, 16873. |
| [42] | Jessop, P. G. In The Handbook of Homogeneous Hydrogenation, Eds.: de Vries, J. G.; Elsevier, C. J., Wiley-VCH, Weinheim, 2007, p. 489. |
| [43] | Tominaga, K.-I.; Sasaki, Y.; Watanabe, T.; Saito, M. J. Chem. Soc., Chem. Commun. 1993, 629. |
| [44] | Huff, C. A.; Sanford, M. S. J. Am. Chem. Soc. 2011, 133, 18122. |
| [45] | Wesselbaum, S.; Stein, T.; Klankermayer, J.; Leitner, W. Angew. Chem., Int. Ed. 2012, 51, 7499. |
| [46] | Rezayee, N. M.; Huff, C. A.; Sanford, M. S. J. Am. Chem. Soc. 2015, 137, 1028. |
| [47] | Wesselbaum, S.; Moha, V.; Meuresch, M.; Brosinski, S.; Thenert, K. M.; Kothe, J.; Stein, T.; Englert, U.; H?lscher, M.; Klankermayer, J.; Leitner, W. Chem. Sci. 2015, 6, 693. |
| [48] | Kothandaraman, J.; Goeppert, A.; Czaun, M.; Olah, G. A.; Prakash, G. K. S. J. Am. Chem. Soc. 2016, 138, 778. |
| [49] | Everett, M.; Wass, D. F. Chem. Commun. 2017, 53, 9502. |
| [50] | Kar, S.; Sen, R.; Kothandaraman, J.; Goeppert, A.; Chowdhury, R.; Munoz, S. B.; Haiges, R.; Prakash, G. K. S. J. Am. Chem. Soc. 2019, 141, 3160. |
| [51] | Kar, S.; Sen, R.; Goeppert, A.; Prakash, G. K. S. J. Am. Chem. Soc. 2018, 140, 1580. |
| [52] | Khusnutdinova, J. R.; Garg, J. A.; Milstein, D. ACS Catal. 2015, 5, 2416. |
| [53] | Ribeiro, A. P. C.; Martins, L. M. D. R. S.; Pombeiro, A. J. L. Green Chem. 2017, 19, 4811. |
| [54] | Lane, E. M.; Zhang, Y.; Hazari, N.; Bernskoetter, W. H. Organometallics 2019, 38, 3084. |
| [55] | Song, Z.; Liu, J.; Bai, Y.; Li, J.; Peng, J. Chin. J. Org. Chem. 2023, 43, 2068. (in Chinese) |
| [55] | (宋姿洁, 刘俊, 白赢, 厉嘉云, 彭家建, 有机化学, 2023, 43, 2068.) |
| [56] | Zhang, Y.; Zhang, T.; Das, S. Green Chem. 2020, 22, 1800. |
| [57] | Goyal, V.; Naik, G.; Narani, A.; Natte, K.; Jagadeesh, R. V. Tetrahedron 2021, 98, 132414. |
| [58] | Fernández-Alvarez, F. J.; Aitanib, A. M.; Oro, L. A. Catal. Sci. Technol. 2014, 4, 611. |
| [59] | Bontemps, S. Coord. Chem. Rev. 2016, 308, 117. |
| [60] | Murphy, L. J.; Hollenhorst, H.; McDonald, R.; Ferguson, M.; Lumsden, M. D.; Turculet, L. Organometallics 2017, 36, 3709. |
| [61] | Chakraborty, S.; Patel, Y. J.; Krause, J. A.; Guan, H. Polyhedron 2012, 32, 30. |
| [62] | Chakraborty, S.; Zhang, J.; Patel, Y. J.; Krause, J. A.; Guan, H. Inorg. Chem. 2013, 52, 37. |
| [63] | Huang, F.; Zhang, C.; Jiang, J.; Wang, Z.-X.; Guan, H. Inorg. Chem. 2011, 50, 3816. |
| [64] | Jin, G.; Werncke, C. G.; Escudié, Y.; Sabo-Etienne, S.; Bontemps, S. J. Am. Chem. Soc. 2015, 137, 9563. |
| [65] | Béthegnies, A.; Escudié, Y.; Nun?ez-Dallos, N.; Vendier, L.; Hurtado, J.; del Rosal, I.; Maron, L.; Bontemps, S. ChemCatChem 2019, 11, 760. |
| [66] | Zhang, D.; Jarava-Barrera, C.; Bontemps, S. ACS Catal. 2021, 11, 4568. |
| [67] | Desmons, S.; Grayson-Steel, K.; Nun?ez-Dallos, N.; Vendier, L.; Hurtado, J.; Clapés, P.; Fauré, R.; Dumon, C.; Bontemps, S. J. Am. Chem. Soc. 2021, 143, 16274. |
| [68] | Desmons, S.; Zhou, Y.; Zhang, D.; Jarava-Barrera, C.; Coffinet, A.; Simonneau, A.; Vendier, L.; Luo, G.; Bontemps, S. Eur. J. Org. Chem. 2023, 26, e2023005. |
| [69] | Aloisi, A.; Berthet, J.-C.; Genre, C.; Thuéry, P.; Cantat, T. Dalton Trans. 2016, 45, 14774. |
| [70] | Lau, S.; Provis-Evans, C. B.; James, A. P.; Webster, R. L. Dalton Trans. 2021, 50, 10696. |
| [71] | Frogneux, X.; Jacquet, O.; Cantat, T. Catal. Sci. Technol. 2014, 4, 1529. |
| [72] | Jurado-Vázquez, T.; García, J. J. Catal. Lett. 2018, 148, 1162. |
| [73] | Li, W.-D.; Zhu, D.-Y.; Li, G.; Chen, J.; Xia, J.-B. Adv. Synth. Catal. 2019, 361, 5098. |
| [74] | Li, W.-D.; Chen, J.; Zhu, D.-Y.; Xia, J.-B. Chin. J. Chem. 2021, 39, 614. |
| [75] | Nylund, P. V. S.; Rigoni, G.; Albrecht, M. Organometallics 2023, 42, 1740. |
| [76] | Xiao, Y.; Xie, F.; Zhang, H.-T.; Zhang, M.-T. JACS Au 2024, 4, 1207. |
| [77] | Li, Y.; Chen, J.-Y.; Zhang, X.; Peng, Z.; Miao, Q.; Chen, W.; Xie, F.; Liao, R.-Z.; Ye, S.; Tung, C.-H.; Wang, W. J. Am. Chem. Soc. 2023, 145, 26915. |
| [78] | Yang, Z.; Shen, C.; Dong, K. Chin. J. Chem. 2022, 40, 2734. |
/
| 〈 |
|
〉 |