

光/镍协同催化实现C(sp3)—H键选择性官能团化
收稿日期: 2024-06-22
修回日期: 2024-07-23
网络出版日期: 2024-08-26
基金资助
国家自然科学基金(22171215); 国家自然科学基金(22301225); 湖北省杰出青年基金(2022CFA092); 湖北省自然科学基金(2023AFB034); 广东省基础与应用基础研究基金(2022A1515010246); 广东省基础与应用基础研究基金(2022A1515110113)
Selective Functionalization of C(sp3)—H Bonds via Photoredox/ Nickel Dual Catalysis
Received date: 2024-06-22
Revised date: 2024-07-23
Online published: 2024-08-26
Supported by
National Natural Science Foundation of China(22171215); National Natural Science Foundation of China(22301225); Hubei Provincial Outstanding Youth Fund(2022CFA092); Hubei Provincial Natural Science Foundation(2023AFB034); Guangdong Basic and Applied Basic Research Foundation(2022A1515010246); Guangdong Basic and Applied Basic Research Foundation(2022A1515110113)
王晓琴 , 许盛 , 平媛媛 , 孔望清 . 光/镍协同催化实现C(sp3)—H键选择性官能团化[J]. 有机化学, 2025 , 45(2) : 383 -422 . DOI: 10.6023/cjoc202406036
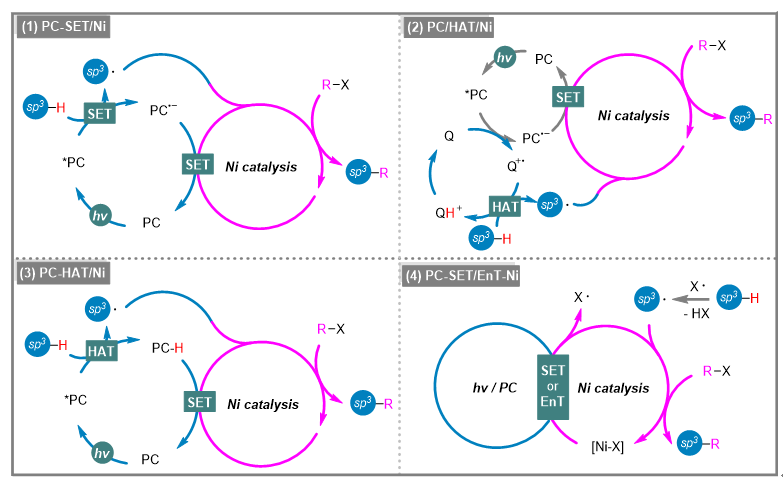
The conversion of readily available hydrocarbons into high value-added chiral compounds via C(sp3)—H functionalization has attracted a great deal of interest from both academia and industry. This transformation is undoubtedly a revolutionary approach due to its inherent atom and step economy, as well as the abundance of starting materials. Classical transition- metal-catalyzed C—H activation usually requires noble metal catalysts, high temperatures, and coordinated directing groups. In recent years, the rapid development of the field of photocatalysis has provided new methods for the activation of inert C(sp3)—H bonds. The combination of photoinduced C(sp3)—H bond cleavage and nickel-catalyzed cross-coupling reactions has emerged as a powerful tool for the selective functionalization of C(sp3)—H bonds. Herein, the recent progress in selective C(sp3)—H functionalization via photoredox/nickel dual catalysis is summarized.
| [1] | Lovering, F. MedChemComm 2013, 4, 515. |
| [2] | Rudolph, A.; Lautens, M. Angew. Chem. Int. Ed. 2009, 48, 2656. |
| [3] | Choi, J.; Fu, G. C. Science 2017, 356, eaaf7230. |
| [4] | Tasker, S. Z.; Standley, E. A.; Jamison, T. F. Nature 2014, 509, 299. |
| [5] | Diccianni, J. B.; Diao, T. Trends Chem. 2019, 1, 830. |
| [6] | Chan, A. Y.; Perry, I. B.; Bissonnette, N. B.; Buksh, B. F.; Edwards, G. A.; Frye, L. I.; Garry, O. L.; Lavagnino, M. N.; Li, B. X.; Liang, Y.; Mao, E.; Millet, A.; Oakley, J. V.; Reed, N. L.; Sakai, H. A.; Seath, C. P.; MacMillan, D. W. C. Chem. Rev. 2022, 122, 1485. |
| [7] | Li, Z.; Li, C.; Ding, Y.; Huo, H., Coord. Chem. Rev. 2022, 460, 214479. |
| [8] | Zuo, Z.; Ahneman, D. T.; Chu, L.; Terrett, J. A.; Doyle, A. G.; MacMillan, D. W. C. Science 2014, 345, 437. |
| [9] | Tellis, J. C.; Primer, D. N.; Molander, G. A. Science 2014, 345, 433. |
| [10] | Corcé, V.; Chamoreau, L.-M.; Derat, E.; Goddard, J.-P.; Ollivier, C.; Fensterbank, L. Angew. Chem. Int. Ed. 2015, 54, 11414. |
| [11] | Gutiérrez-Bonet, á.; Tellis, J. C.; Matsui, J. K.; Vara, B. A.; Molander, G. A. ACS Catal. 2016, 6, 8004. |
| [12] | Zhang, X.; MacMillan, D. W. C. J. Am. Chem. Soc. 2016, 138, 13862. |
| [13] | Vara, B. A.; Patel, N. R.; Molander, G. A. ACS Catal. 2017, 7, 3955. |
| [14] | Smith, R. T.; Zhang, X.; Rincón, J. A.; Agejas, J.; Mateos, C.; Barberis, M.; García-Cerrada, S.; de Frutos, O.; MacMillan, D. W. C. J. Am. Chem. Soc. 2018, 140, 17433 |
| [15] | Dong, Z.; MacMillan, D. W. C. Nature 2021, 598, 451. |
| [16] | Prier, C. K.; Rankic, D. A.; MacMillan, D. W. C. Chem. Rev. 2013, 113, 5322. |
| [17] | Romero, N. A.; Nicewicz, D. A. Chem. Rev. 2016, 116, 10075. |
| [18] | Bellotti, P.; Huang, H.-M.; Faber, T.; Glorius, F. Chem. Rev. 2023, 123, 4237. |
| [19] | Qin, Q.; Jiang, H.; Hu, Z.; Ren, D.; Yu, S. Chem. Rec. 2017, 17, 754. |
| [20] | Cao, H.; Tang, X.; Tang, H.; Yuan, Y.; Wu, J. Chem Catal. 2021, 1, 523. |
| [21] | Capaldo, L.; Ravelli, D.; Fagnoni, M. Chem. Rev. 2022, 122, 1875. |
| [22] | Zhang, J.; Rueping, M. Chem. Soc. Rev. 2023, 52, 4099. |
| [23] | McNally, A.; Prier, C. K.; MacMillan, D. W. C. Science 2011, 334, 1114. |
| [24] | Ahneman, D. T.; Doyle, A. G. Chem. Sci. 2016, 7, 7002. |
| [25] | Gui, Y.-Y.; Wang, Z.-X.; Zhou, W.-J.; Liao, L.-L.; Song, L.; Yin, Z.-B.; Li, J.; Yu, D.-G. Asian J. Org. Chem. 2018, 7, 537. |
| [26] | Gui, Y.-Y.; Liao, L.-L.; Sun, L.; Zhang, Z.; Ye, J.-H.; Shen, G.; Lu, Z.-P.; Zhou, W.-J.; Yu, D.-G. Chem. Commun. 2017, 53, 1192. |
| [27] | Joe, C. L.; Doyle, A. G. Angew. Chem. Int. Ed. 2016, 55, 4040. |
| [28] | Kim, W.; Koo, J.; Lee, H. G. Chem. Sci. 2021, 12, 4119. |
| [29] | Cheng, C.; Lv, G.-F.; Wu, S.; Li, Y.; Li, J.-H. Org. Lett. 2023, 25, 4236. |
| [30] | Liu, K.; Studer, A. Angew. Chem. Int. Ed. 2022, 61, e202206533. |
| [31] | Cannalire, R.; Pelliccia, S.; Sancineto, L.; Novellino, E.; Tron, G. C.; Giustiniano, M. Chem. Soc. Rev. 2021, 50, 766. |
| [32] | Rosemann, N. W.; Eu?ner, J. P.; Beyer, A.; Koch, S. W.; Volz, K.; Dehnen, S.; Chatterjee, S. Science 2016, 352, 1301. |
| [33] | Maity, B.; Zhu, C.; Yue, H.; Huang, L.; Harb, M.; Minenkov, Y.; Rueping, M.; Cavallo, L. J. Am. Chem. Soc. 2020, 142, 16942. |
| [34] | Twilton, J.; Christensen, M.; DiRocco, D. A.; Ruck, R. T.; Davies, I. W.; MacMillan, D. W. C. Angew. Chem. Int. Ed. 2018, 57, 5369. |
| [35] | Gui, Y.-Y.; Chen, X.-W.; Zhou, W.-J.; Yu, D.-G. Synlett 2017, 28, 2581. |
| [36] | Le, C.; Liang, Y.; Evans, R. W.; Li, X.; MacMillan, D. W. C. Nature 2017, 547, 79. |
| [37] | Ma, Z.-Y.; Li, M.; Guo, L.-N.; Liu, L.; Wang, D.; Duan, X.-H. Org. Lett. 2021, 23, 474. |
| [38] | Dong, M.; Jia, Y.; Zhou, W.; Gao, J.; Lv, X.; Luo, F.; Zhang, Y.; Liu, S. Org. Chem. Front. 2021, 8, 6881. |
| [39] | Chu, J. C. K.; Rovis, T. Nature 2016, 539, 272. |
| [40] | Choi, G. J.; Zhu, Q.; Miller, D. C.; Gu, C. J.; Knowles, R. R. Nature 2016, 539, 268. |
| [41] | Thullen, S. M.; Treacy, S. M.; Rovis, T. J. Am. Chem. Soc. 2019, 141, 14062. |
| [42] | Xu, B.; Tambar, U. K. ACS Catal. 2019, 9, 4627. |
| [43] | Kanegusuku, A. L. G.; Castanheiro, T.; Ayer, S. K.; Roizen, J. L. Org. Lett. 2019, 21, 6089. |
| [44] | Simons, R. T.; Nandakumar, M.; Kwon, K.; Ayer, S. K.; Venneti, N. M.; Roizen, J. L. J. Am. Chem. Soc. 2023, 145, 3882. |
| [45] | Zhang, P.; Le, C. C.; MacMillan, D. W. C. J. Am. Chem. Soc. 2016, 138, 8084. |
| [46] | Wang, Z.; Ji, X.; Han, T.; Deng, G.-J.; Huang, H. Adv. Synth. Catal. 2019, 361, 5643. |
| [47] | Rand, A. W.; Yin, H.; Xu, L.; Giacoboni, J.; Martin-Montero, R.; Romano, C.; Montgomery, J.; Martin, R. ACS Catal. 2020, 10, 4671. |
| [48] | Yue, W.-J.; Day, C. S.; Martin, R. J. Am. Chem. Soc. 2021, 143, 6395. |
| [49] | Rand, A. W.; Chen, M.; Montgomery, J. Chem. Sci. 2022, 13, 10566. |
| [50] | Kawasaki, T.; Ishida, N.; Murakami, M. J. Am. Chem. Soc. 2020, 142, 3366. |
| [51] | Griffiths, O. M.; Esteves, H. A.; Chen, Y.; Sowa, K.; May, O. S.; Morse, P.; Blakemore, D. C.; Ley, S. V. J. Org. Chem. 2021, 86, 13559. |
| [52] | Ishida, N.; Son, M.; Kawasaki, T.; Ito, M.; Murakami, M. Synlett 2021, 32, 2067. |
| [53] | Ishida, N.; Shinoya, H.; Kamino, Y.; Kawasaki, T.; Murakami, M. Chem. Lett. 2022, 51, 765. |
| [54] | Kawasaki, T.; Tosaki, T.; Ishida, N.; Murakami, M. Org. Lett. 2021, 23, 7683. |
| [55] | Kawasaki, T.; Tosaki, T.; Miki, S.; Takada, T.; Murakami, M.; Ishida, N. J. Am. Chem. Soc. 2024, 146, 17566. |
| [56] | Wang, Q.-L.; Huang, H.; Mao, G.; Deng, G.-J. Green Chem. 2022, 24, 8324. |
| [57] | Kawasaki, T.; Yamazaki, K.; Tomono, R.; Ishida, N.; Murakami, M. Chem. Lett. 2021, 50, 1684. |
| [58] | Huang, L.; Szewczyk, M.; Kancherla, R.; Maity, B.; Zhu, C.; Cavallo, L.; Rueping, M. Nat. Commun. 2023, 14, 548. |
| [59] | Yi, H.; Zhang, G.; Wang, H.; Huang, Z.; Wang, J.; Singh, A. K.; Lei, A. Chem. Rev. 2017, 117, 9016. |
| [60] | Scaiano, J. C.; Wubbels, G. G. J. Am. Chem. Soc. 1981, 103, 640. |
| [61] | Vasilopoulos, A.; Krska, S. W.; Stahl, S. S. Science 2021, 372, 398. |
| [62] | Gong, Y.; Su, L.; Zhu, Z.; Ye, Y.; Gong, H. Angew. Chem. Int. Ed. 2022, 61, e202201662. |
| [63] | Chen, M.; Ventura, A. M.; Das, S.; Ibrahim, A. F.; Zimmerman, P. M.; Montgomery, J. J. Am. Chem. Soc. 2023, 145, 20176. |
| [64] | Kato, S.; Saga, Y.; Kojima, M.; Fuse, H.; Matsunaga, S.; Fukatsu, A.; Kondo, M.; Masaoka, S.; Kanai, M. J. Am. Chem. Soc. 2017, 139, 2204. |
| [65] | Huang, H.-M.; Bellotti, P.; Chen, P.-P.; Houk, K. N.; Glorius, F. Nat. Synth. 2022, 1, 59. |
| [66] | Ravelli, D.; Fagnoni, M.; Fukuyama, T.; Nishikawa, T.; Ryu, I. ACS Catal. 2018, 8, 701. |
| [67] | Perez-Prieto, J.; Galian, R.; Miranda, M. Mini-Rev. Org. Chem 2006, 3, 117. |
| [68] | Waele, V. D.; Poizat, O.; Fagnoni, M.; Bagno, A.; Ravelli, D. ACS Catal. 2016, 6, 7174. |
| [69] | Perry, I. B.; Brewer, T. F.; Sarver, P. J.; Schultz, D. M.; DiRocco, D. A.; MacMillan, D. W. C. Nature 2018, 560, 70. |
| [70] | Mazzarella, D.; Pulcinella, A.; Bovy, L.; Broersma, R.; No?l, T. Angew. Chem. Int. Ed. 2021, 60, 21277. |
| [71] | Liu, W.; Ke, Y.; Liu, C.; Kong, W. Chem. Commun. 2022, 58, 11937. |
| [72] | Jin, Y.; Ng, E. W. H.; Fan, T.; Hirao, H.; Gong, L.-Z. ACS Catal. 2022, 12, 10039. |
| [73] | Pilli, R.; Selvam, K.; Balamurugan, B. S. S.; Jose, V.; Rasappan, R. Org. Lett. 2024, 26, 2993. |
| [74] | Hu, Z.; Wang, D.; Xu, T. ACS Catal. 2024, 14, 547. |
| [75] | Bour, J. R.; Ferguson, D. M.; McClain, E. J.; Kampf, J. W.; Sanford, M. S. J. Am. Chem. Soc. 2019, 141, 8914. |
| [76] | Tsymbal, A. V.; Bizzini, L. D.; MacMillan, D. W. C. J. Am. Chem. Soc. 2022, 144, 21278. |
| [77] | Mao, E.; MacMillan, D. W. C. J. Am. Chem. Soc. 2023, 145, 2787. |
| [78] | Wang, Q.; Ni, S.; Yu, L.; Pan, Y.; Wang, Y. ACS Catal. 2022, 12, 11071. |
| [79] | Zhou, S.; Liu, T.; Bao, X. J. Catal. 2022, 415, 142. |
| [80] | Xu, L., F., W., Chen, F., Zhu, S., Chu, L. Chin. J. Org. Chem. 2022, 42, 1. |
| [81] | Xu, S.; Chen, H.; Zhou, Z.; Kong, W. Angew. Chem. Int. Ed. 2021, 60, 7405. |
| [82] | Wang, D.; Ackermann, L. Chem. Sci. 2022, 13, 7256. |
| [83] | Liu, W.; Liu, C.; Wang, M.; Kong, W. ACS Catal. 2022, 12, 10207. |
| [84] | Wagner, P. J.; Kemppainen, A. E.; Schott, H. N. J. Am. Chem. Soc. 1970, 92, 5280. |
| [85] | Péter, á.; Agasti, S.; Knowles, O.; Pye, E.; Procter, D. J. Chem. Soc. Rev. 2021, 50, 5349. |
| [86] | Shen, Y.; Gu, Y.; Martin, R. J. Am. Chem. Soc. 2018, 140, 12200. |
| [87] | Dewanji, A.; Krach, P. E.; Rueping, M. Angew. Chem. Int. Ed. 2019, 58, 3566. |
| [88] | Krach, P. E.; Dewanji, A.; Yuan, T.; Rueping, M. Chem. Commun. 2020, 56, 6082. |
| [89] | Ren, C.-C.; Wang, T.-Q.; Zhang, Y.; Peng, D.; Liu, X.-Q.; Wu, Q.-A.; Liu, X.-F.; Luo, S.-P. ChemistrySelect 2021, 6, 2523. |
| [90] | Ishida, N.; Masuda, Y.; Imamura, Y.; Yamazaki, K.; Murakami, M. J. Am. Chem. Soc. 2019, 141, 19611. |
| [91] | Xu, Z.; Liu, D.; Yu, H.; Ahlquist, M. S. G.; Fu, Y. Mol. Catal. 2021, 514, 111785. |
| [92] | Campbell, M. W.; Yuan, M.; Polites, V. C.; Gutierrez, O.; Molander, G. A. J. Am. Chem. Soc. 2021, 143, 3901. |
| [93] | Zhang, L.; Si, X.; Yang, Y.; Zimmer, M.; Witzel, S.; Sekine, K.; Rudolph, M.; Hashmi, A. S. K. Angew. Chem. Int. Ed. 2019, 58, 1823. |
| [94] | Si, X.; Zhang, L.; Hashmi, A. S. K. Org. Lett. 2019, 21, 6329. |
| [95] | Heitz, D. R.; Tellis, J. C.; Molander, G. A. J. Am. Chem. Soc. 2016, 138, 12715. |
| [96] | Ishida, N.; Masuda, Y.; Ishikawa, N.; Murakami, M. Asian J. Org. Chem. 2017, 6, 669. |
| [97] | Huang, L.; Rueping, M. Angew. Chem. Int. Ed. 2018, 57, 10333. |
| [98] | Santos, M. S.; Corrêa, A. G.; Paix?o, M. W.; K?nig, B. Adv. Synth. Catal. 2020, 362, 2367. |
| [99] | Kancherla, R.; Muralirajan, K.; Maity, B.; Karuthedath, S.; Kumar, G. S.; Laquai, F.; Cavallo, L.; Rueping, M. Nat. Commun. 2022, 13, 2737. |
| [100] | Shields, B. J.; Doyle, A. G. J. Am. Chem. Soc. 2016, 138, 12719. |
| [101] | Nielsen, M. K.; Shields, B. J.; Liu, J.; Williams, M. J.; Zacuto, M. J.; Doyle, A. G. Angew. Chem. Int. Ed. 2017, 56, 7191. |
| [102] | Cai, Y.; Tang, Y.; Fan, L.; Lefebvre, Q.; Hou, H.; Rueping, M. ACS Catal. 2018, 8, 9471. |
| [103] | Das, S.; Murugesan, K.; Villegas Rodríguez, G. J.; Kaur, J.; Barham, J. P.; Savateev, A.; Antonietti, M.; K?nig, B. ACS Catal. 2021, 11, 1593. |
| [104] | Deng, H.-P.; Fan, X.-Z.; Chen, Z.-H.; Xu, Q.-H.; Wu, J. J. Am. Chem. Soc. 2017, 139, 13579. |
| [105] | Go, S. Y.; Lee, G. S.; Hong, S. H. Org. Lett. 2018, 20, 4691. |
| [106] | Ackerman, L. K. G.; Martinez Alvarado, J. I.; Doyle, A. G. J. Am. Chem. Soc. 2018, 140, 14059. |
| [107] | Sun, Z.; Kumagai, N.; Shibasaki, M. Org. Lett. 2017, 19, 3727. |
| [108] | Lee, G. S.; Won, J.; Choi, S.; Baik, M.-H.; Hong, S. H. Angew. Chem. Int. Ed. 2020, 59, 16933. |
| [109] | Zhang, J.; Niu, Y.; Kong, F.; Yan, M. J. Catal. 2022, 416, 58. |
| [110] | Lee, G. S.; Park, B.; Hong, S. H. Nat. Commun. 2022, 13, 5200. |
| [111] | Xu, G.-Q.; Wang, W. D.; Xu, P.-F. J. Am. Chem. Soc. 2024, 146, 1209. |
| [112] | Cheng, X.; Lu, H.; Lu, Z. Nat. Commun. 2019, 10, 3549. |
| [113] | Zhang, W.; Shu, X.; Huan, L.; Cheng, B.; Huo, H. Org. Biomol. Chem. 2021, 19, 9407. |
| [114] | Cheng, X.; Li, T.; Liu, Y.; Lu, Z. ACS Catal. 2021, 11, 11059. |
| [115] | Xu, J.; Li, Z.; Xu, Y.; Shu, X.; Huo, H. ACS Catal. 2021, 11, 13567. |
| [116] | Shu, X.; Zhong, D.; Lin, Y.; Qin, X.; Huo, H. J. Am. Chem. Soc. 2022, 144, 8797. |
| [117] | Xu, S.; Ping, Y.; Li, W.; Guo, H.; Su, Y.; Li, Z.; Wang, M.; Kong, W. J. Am. Chem. Soc. 2023, 145, 5231. |
| [118] | Shu, X.; Huan, L.; Huang, Q.; Huo, H. J. Am. Chem. Soc. 2020, 142, 19058. |
| [119] | Huan, L.; Shu, X.; Zu, W.; Zhong, D.; Huo, H. Nat. Commun. 2021, 12, 3536. |
| [120] | Shu, X.; Zhong, D.; Huang, Q.; Huan, L.; Huo, H. Nat. Commun 2023, 14, 125. |
| [121] | Cuesta-Galisteo, S.; Sch?rgenhumer, J.; Hervieu, C. Nevado, C. Angew. Chem. Int. Ed. 2024, 63, e202313717. |
| [122] | Li, J.; Cheng, B.; Shu, X.; Xu, Z.; Li, C.; Huo, H. J. Am. Chem. Soc. 2024, 146, 19909. |
| [123] | Hu, X.; Cheng-Sánchez, I.; Kong, W.; Molander, G. A. Nevado, C., Nat. Catal. 2024, 7, 655. |
/
| 〈 |
|
〉 |