

以CO2为C1源合成N-芳基吡咯烷
收稿日期: 2024-05-29
修回日期: 2024-08-22
网络出版日期: 2024-09-10
基金资助
国家重点研发计划(2023YFA1506404); 甘肃省科技计划(23ZDFA003); 甘肃省科技计划(23JRRA1144); 甘肃省科技计划(23JRRA1028); 甘肃省科技计划(23CXGA0043); 兰州市科技计划(2024-1-17); 兰州市科技计划(2023-1-17); 兰州市科技计划(2023-QN-18); 中央高校基本科研业务专项(lzujbky-2022-sp09); 中央高校基本科研业务专项(lzujbky-2023-ct02); 中央高校基本科研业务专项(lzujbky-2023-pd08); 陇药协同创新中心和甘肃省药物研发计(2022GSMPA0010)
Synthesis of N-Arylpyrrolidines Using CO2 as C1 Source
Received date: 2024-05-29
Revised date: 2024-08-22
Online published: 2024-09-10
Supported by
National Key R&D Program of China(2023YFA1506404); Science and Technology Program of Gansu Province(23ZDFA003); Science and Technology Program of Gansu Province(23JRRA1144); Science and Technology Program of Gansu Province(23JRRA1028); Science and Technology Program of Gansu Province(23CXGA0043); Lanzhou Science and Technology Planning Project(2024-1-17); Lanzhou Science and Technology Planning Project(2023-1-17); Lanzhou Science and Technology Planning Project(2023-QN-18); Fundamental Research Funds for the Central Universities(lzujbky-2022-sp09); Fundamental Research Funds for the Central Universities(lzujbky-2023-ct02); Fundamental Research Funds for the Central Universities(lzujbky-2023-pd08); Collaborative Innovation Center for Northwestern Chinese Medicine of Lanzhou University and the Drug Research Project of Gansu Province(2022GSMPA0010)
周于佳 , 孔荞 , 朱道勇 , 王少华 . 以CO2为C1源合成N-芳基吡咯烷[J]. 有机化学, 2024 , 44(10) : 3185 -3197 . DOI: 10.6023/cjoc202405042
A series of N-arylpyrrolidines were synthesized by using carbon dioxide and N-arylpropylamine derivatives as substrates, N-heterocyclic carbene IPr as catalyst, 1,3,5,7-tetramethylcyclotetrasiloxane (TMCTS) as reducing agent, triethylenediamine as base at atmospheric pressure. The reaction features mild conditions, metal-free and good functional group tolerance.
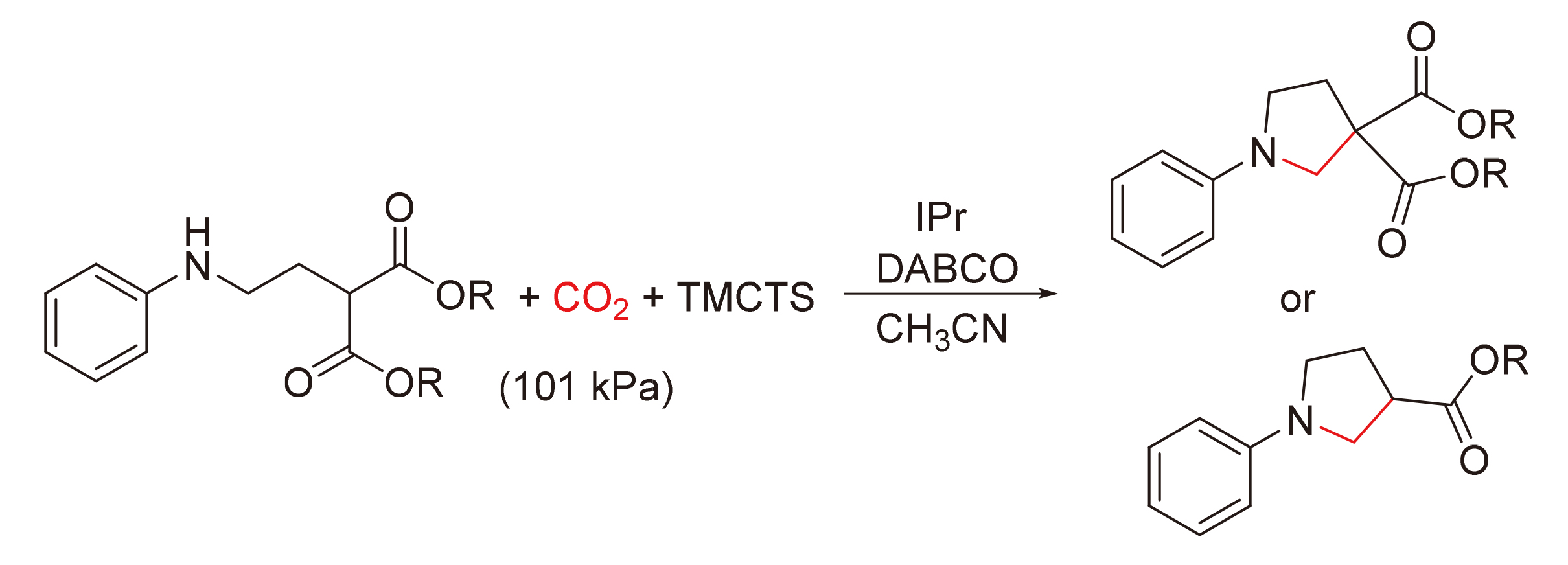
Key words: CO2; N-arylpropylamine; N-arylpyrrolidine
| [1] | Balaban, A. T.; Oniciu, D. C.; Katritzky, A. R. Chem. Rev. 2004, 104, 2777. |
| [2] | La Regina, G.; Silvestri, R.; Artico, M.; Lavecchia, A.; Novellino, E.; Befani, O.; Turini, P.; Agostinelli, E. J. Med. Chem. 2007, 50, 922. |
| [3] | Pinder, A. R. Nat. Prod. Rep. 1992, 9, 17. |
| [4] | Srinivasan, V.; Panneerselvam, M.; Pavithra, N.; Anandan, S.; Sundaravel, K.; Jaccob, M.; Kathiravan, A. J. Photochem. Photobiol. A 2017, 332, 453. |
| [5] | Lei, Y.-R.; Xiong, T.-K.; Yu, X.-T.; Huang, X.-B.; Tang, X.-L.; Yi, H.-H.; Zhou, Y.-S.; Zhao, S.-Z.; Sun, L.; Gao, F.-Y. Acta Chim. Sinica 2024, 82, 396. (in Chinese) |
| [5] | (雷雅茹, 熊廷楷, 于湘涛, 黄秀兵, 唐晓龙, 易红宏, 周远松, 赵顺征, 孙龙, 高凤雨, 化学学报, 2024, 82, 396.) |
| [6] | Cui, G.-Q.; Hu, Y.-Y.; Lou, Y.-J.; Zhou, M.-X.; Li, Y.-M.; Wang, Y.-J.; Jiang, G.-Y.; Xu, C.-M. Acta Chim. Sinica 2023, 81, 1081. (in Chinese) |
| [6] | (崔国庆, 胡溢玚, 娄颖洁, 周明霞, 李宇明, 王雅君, 姜桂元, 徐春明, 化学学报, 2023, 81, 1081.) |
| [7] | Jiang, Y.-L.; Li, G.-C.; Chen, Q.-S.; Xu, Z.-N.; Lin, S.-S.; Guo, G.-C. Acta Chim. Sinica 2022, 80, 703. (in Chinese) |
| [7] | (蒋银龙, 李国超, 陈青松, 徐忠宁, 林姗姗, 郭国聪, 化学学报, 2022, 80, 703.) |
| [8] | Song, Y.-P.; Yin, K.; Chen, Y.-J.; Zhao, B.; Zhang, Y.; Zhu, X.-H.; Yuan, D.; Yao, Y.-M. Chin. J. Chem. 2023, 41, 805. |
| [9] | Wei, Z.-M.; Liu, Y.-H.; Ding, J.; He, Q.-Y.; Zhang, Q.; Zhai, Y.-M. Chin. J. Chem. 2023, 41, 3553. |
| [10] | Liu, J.-Y.; Li, P.-S.; Bi, J.-H.; Wang, Y.; Zhu, Q.-G.; Sun, X.-F.; Zhang, J.-L.; Liu, Z.-M.; Han, B.-X. Chin. J. Chem. 2023, 41, 1443. |
| [11] | Hubbard, H.; Lawitz, E. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 9. |
| [12] | Popovici-Muller, J.; Lemieux, R. M.; Artin, E.; Saunders, J. O.; Salituro, F. G.; Travins, J.; Cianchetta, G.; Cai, Z. W.; Zhou, D.; Cui, D. W.; Chen, P.; Straley, K.; Tobin, E.; Wang, F.; David, M. D.; Penard-Lacronique, V.; Quivoron, C.; Saada, V.; Botton, S.; Gross, S.; Dang, L.; Yang, H.; Utley, L.; Chen, Y.; Kim, H.; Jin, S. F.; Gu, Z. W.; Yao, G.; Luo, Z. Y.; Lv, X. B.; Fang, C.; Yan, L. P.; Olaharski, A.; Silverman, L.; Biller, S.; Su, S. M.; Yen, K. ACS Med. Chem. Lett. 2018, 9, 300. |
| [13] | Vangapandu, H. V.; Jain, N.; Gandhi, V. Expert Opin. Invest. Drugs 2017, 26, 625. |
| [14] | Sinkule, J. A. Pharmacotherapy 1984, 4, 61. |
| [15] | Sarma, M.; Chatterjee, T.; Ghanta, S.; Daset, S. K. J. Org. Chem. 2012, 77, 432. |
| [16] | Cui, X.-J.; Dai, X.-C.; Deng, Y.-Q.; Shi, F. Chem.-Eur. J. 2013, 19, 3665. |
| [17] | Watanabe, Y.; Shim, S. C.; Uchida, H.; Mitsudo, T.; Takegami, Y. Tetrahedron 1979, 35, 1433. |
| [18] | Cano, R.; Ramón, D. J.; Yus, M. J. Org. Chem. 2011, 76, 654. |
| [19] | Korbad, B. L.; Lee, S. H. Chem. Commun. 2014, 50, 8985. |
| [20] | Tominaga, H. Int. J. Soc. Mater. Eng. Resour. 1995, 3, 33. |
| [21] | Liu, Q.; Wu, L.; Jackstell, R.; Beller, M. Nat. Commun. 2015, 6, 5933. |
| [22] | Klankermayer, J.; Wesselbaum, S.; Beydoun, K.; Leitner, W. Angew. Chem., Int. Ed. 2016, 55, 7296. |
| [23] | Zhang, L.-L.; Han, Z.-B.; Zhang, L.; Li, M.-X.; Ding, K.-L. Chin. J. Org. Chem. 2016, 36, 1824. (in Chinese) |
| [23] | (张琳莉, 韩召斌, 张磊, 李明星, 丁奎岭, 有机化学, 2016, 36, 1824.) |
| [24] | Tlili, A.; Blondiaux, E.; Frogneux, X.; Cantat, T. Green Chem. 2015, 17, 157. |
| [25] | Li, Y.; Cui, X.; Dong, K.; Junge, K.; Beller, M. ACS Catal. 2017, 7, 1077. |
| [26] | Cabrero-Antonino, J. R.; Adam, R.; Beller, M. Angew. Chem., Int. Ed. 2019, 58, 12820. |
| [27] | Zhang, Y.; Zhang, T.; Das, S. Green Chem. 2020, 22, 1800. |
| [28] | Huang, K.; Sun, C.-L.; Shi, Z.-J. Chem. Soc. Rev. 2011, 40, 2435. |
| [29] | Zhang, W.-X.; Liao, P.-Q.; Lin, R.-B.; Wei, Y.-S.; Zeng, M.-H.; Chen, X.-M. Coord. Chem. Rev. 2015, 293-294, 263. |
| [30] | Zhang, H.; Sun, H.-J.; Li, X.-Y. Chin. J. Org. Chem. 2016, 36, 2843. (in Chinese) |
| [30] | (仉花, 孙宏建, 李晓燕, 有机化学, 2016, 36, 2843.) |
| [31] | Zhang, W.-Z.; Zhang, N.; Guo, C.-X.; Lü, X.-B. Chin. J. Org. Chem. 2017, 37, 1309. (in Chinese) |
| [31] | (张文珍, 张宁, 郭春晓, 吕小兵, 有机化学, 2017, 37, 1309.) |
| [32] | Chen, J.; McGraw, M.; Chen, E. Y.-X. ChemSusChem. 2019, 12, 4543. |
| [33] | Wang, X.; Xia, C.; Wu, L. Green Chem. 2018, 20, 5415. |
| [34] | Duan, D.-S.; Ma, Y.; Liu, Y.-B.; Cheng, F.; Zhu, D.-Y.; Wang, S.-H. Chin. J. Org. Chem. 2024, 44, 1675. (in Chinese) |
| [34] | (段东森, 马媛, 刘宇博, 程富, 朱道勇, 王少华, 有机化学, 2024, 44, 1675.) |
| [35] | Li, B.; Sortais, J.-B.; Darcel, C. RSC Adv. 2016, 6, 57603. |
| [36] | Addis, D.; Das, S.; Junge, K.; Beller, M. Angew. Chem., Int. Ed. 2011, 50, 6004. |
| [37] | Ostapowicz, T. G.; Schmitz, M.; Krystof, M.; Klankermayer, J.; Leitner, W. Angew. Chem., Int. Ed. 2013, 52, 12119. |
| [38] | Wu, L.; Liu, Q.; Fleischer, I.; Jackstell, R.; Beller, M. Nat. Commun. 2014, 5, 3091. |
| [39] | Ren, X.; Zheng, Z.; Zhang, L.; Wang, Z.; Xia, C.; Ding, K. Angew. Chem., Int. Ed. 2017, 56, 310. |
| [40] | Guo, Z.; Yang, B.; Jia, J.; Wei, X. Asian. J. Org. Chem. 2023, 12, e202300097. |
| [41] | Thenert, K.; Beydoun, K.; Wiesenthal, J.; Leitner, W.; Klankermayer, J. Angew. Chem., Int. Ed. 2016, 55, 12266. |
| [42] | Jin, G.; Werncke, C. G.; Escudié, Y.; Sabo-Etienne, S.; Bontemps, S. J. Am. Chem. Soc. 2015, 137, 9563. |
| [43] | Frogneux, X.; Blondiaux, E.; Thuéry, P.; Cantat, T. ACS Catal. 2015, 5, 3983. |
| [44] | Liu, M.; Qin, T.; Zhang, Q.; Fang, C.; Fu, Y.; Lin, B.-L. Sci. China: Chem. 2015, 58, 1524. |
| [45] | Yu, Z.; Li, Z. Y.; Zhang, L. L.; Zhu, K. X.; Wu, H. G.; Li, H.; Yang, S. Green Chem. 2021, 23, 5759. |
| [46] | Zhu, D.-Y.; Fang, L.; Han, H.; Wang, Y.; Xia, J.-B. Org. Lett. 2017, 19, 4259. |
| [47] | Li, W.; Chen, J.; Zhu, D.; Xia, J. Chin. J. Chem. 2021, 39, 614. |
| [48] | Miles, D. H.; Huang, B.-S. J. Org. Chem. 1976, 41, 208. |
| [49] | Nusse, R.; Clevers, H. Cell 2017, 169, 985. |
| [50] | Kakugawa, S.; Langton, P.; Zebisch, M.; Howell, S.; Chang, T.; Liu, Y.; Feizi, T.; Bineva, G.; O'Reilly, N.; Snijders, A.; Jones, E.; Vincent, J. Nature 2015, 519, 187. |
| [51] | Zhang, X.; Cheong, S.; Amado, N.; Reis, A.; MacDonald, B.; Zebisch, M.; Jones, E.; Abreu, J.; He, X. Dev. Cell 2015, 32, 719. |
| [52] | Mahy, W.; Patel, M.; Steadman, D.; Woodward, H. L.; Atkinson, B. N.; Svensson, F.; Willis, N. J.; Flint, A.; Papatheodorou, D.; Zhao, Y.; Vecchia, L.; Ruza, R. R.; Hillier, J.; Frew, S.; Monaghan, A.; Costa, A.; Bictash, M.; Walter, M. W.; Jones, E. Y.; Fish, P. V. J. Med. Chem. 2020, 63, 9464. |
| [53] | Zhu, D.-Y.; Wang, S.-H. CN 202311774883, 7, 2023. |
/
| 〈 |
|
〉 |