有机化学 ›› 2024, Vol. 44 ›› Issue (10): 3185-3197.DOI: 10.6023/cjoc202405042 上一篇 下一篇
所属专题: 二氧化碳专题合集
研究论文
收稿日期:2024-05-29
修回日期:2024-08-22
发布日期:2024-09-10
基金资助:
Yujia Zhou, Qiao Kong, Daoyong Zhu*( ), Shaohua Wang*(
), Shaohua Wang*( )
)
Received:2024-05-29
Revised:2024-08-22
Published:2024-09-10
Contact:
*E-mail: Supported by:文章分享
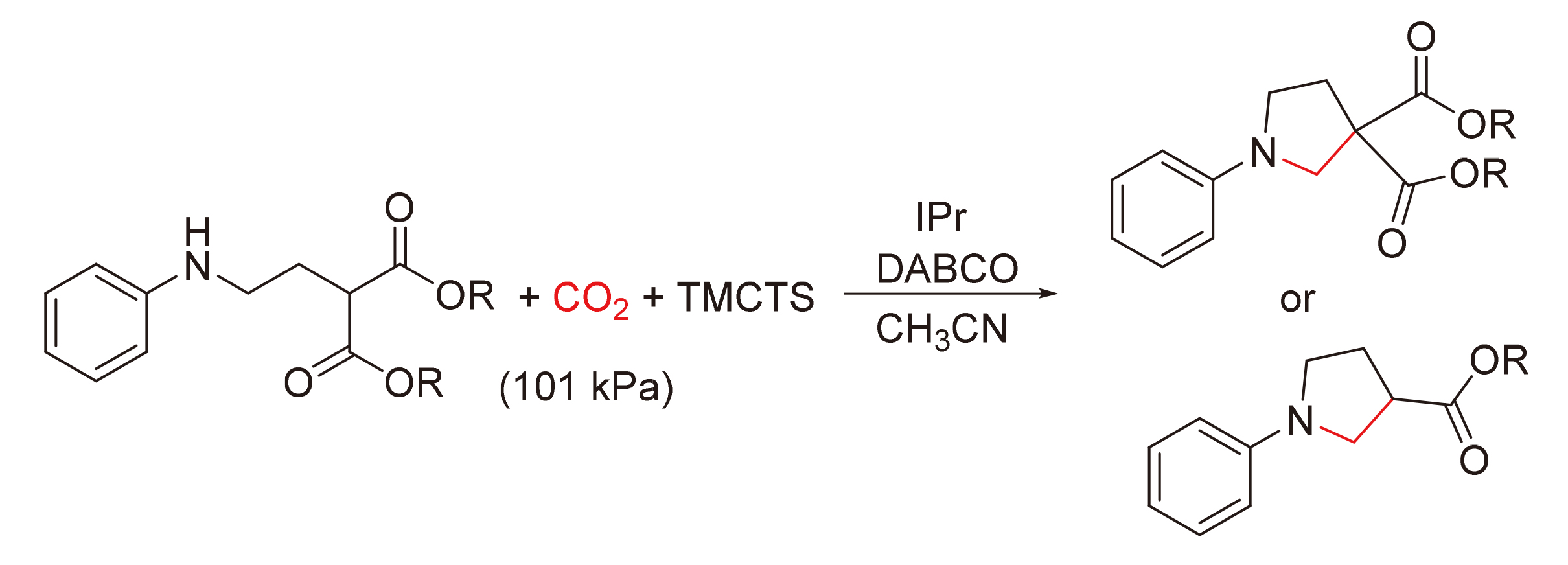
在标准大气压下, 以二氧化碳和N-芳基丙基胺衍生物为原料, N-杂环卡宾IPr为催化剂, 1,3,5,7-四甲基环四硅氧烷(TMCTS)为还原剂, 三乙烯二胺为碱, 发展了合成系列N-芳基吡咯烷类化合物的新方法. 该反应条件温和, 无金属催化剂参与, 且反应具有良好的官能团耐受性.
周于佳, 孔荞, 朱道勇, 王少华. 以CO2为C1源合成N-芳基吡咯烷[J]. 有机化学, 2024, 44(10): 3185-3197.
Yujia Zhou, Qiao Kong, Daoyong Zhu, Shaohua Wang. Synthesis of N-Arylpyrrolidines Using CO2 as C1 Source[J]. Chinese Journal of Organic Chemistry, 2024, 44(10): 3185-3197.
| Entry | Solvent | Reductant | Catalyst | Base | Yield/% |
|---|---|---|---|---|---|
| 1 | CH3CN | PhSiH3 | IPr | — | n.d. |
| 2 | CH3CN | PhSiH3 | — | K2CO3 | n.d. |
| 3 | CH3CN | PhSiH3 | TBD | K2CO3 | 33 |
| 4 | CH3CN | PhSiH3 | IPr | K2CO3 | 50 |
| 5 | CH3CN | PhSiH3 | DBU | K2CO3 | 17 |
| 6 | CH3CN | PhSiH3 | IPr | NaOH | 8 |
| 7 | CH3CN | PhSiH3 | IPr | DMAP | 10 |
| 8 | CH3CN | PhSiH3 | IPr | TBD | 16 |
| 9 | CH3CN | PhSiH3 | IPr | DABCO | 60 |
| 10 | CH3CN | (EtO)3SiH | IPr | DABCO | n.d. |
| 11 | CH3CN | Me(EtO)2SiH | IPr | DABCO | n.d. |
| 12 | CH3CN | PMHS | IPr | DABCO | 67 |
| 13 | CH3CN | TMCTS | IPr | DABCO | 82 |
| 14 | THF | TMCTS | IPr | DABCO | 54 |
| 15 | DCE | TMCTS | IPr | DABCO | 10 |
| 16 | Butyronitrile | TMCTS | IPr | DABCO | 72 |
| Entry | Solvent | Reductant | Catalyst | Base | Yield/% |
|---|---|---|---|---|---|
| 1 | CH3CN | PhSiH3 | IPr | — | n.d. |
| 2 | CH3CN | PhSiH3 | — | K2CO3 | n.d. |
| 3 | CH3CN | PhSiH3 | TBD | K2CO3 | 33 |
| 4 | CH3CN | PhSiH3 | IPr | K2CO3 | 50 |
| 5 | CH3CN | PhSiH3 | DBU | K2CO3 | 17 |
| 6 | CH3CN | PhSiH3 | IPr | NaOH | 8 |
| 7 | CH3CN | PhSiH3 | IPr | DMAP | 10 |
| 8 | CH3CN | PhSiH3 | IPr | TBD | 16 |
| 9 | CH3CN | PhSiH3 | IPr | DABCO | 60 |
| 10 | CH3CN | (EtO)3SiH | IPr | DABCO | n.d. |
| 11 | CH3CN | Me(EtO)2SiH | IPr | DABCO | n.d. |
| 12 | CH3CN | PMHS | IPr | DABCO | 67 |
| 13 | CH3CN | TMCTS | IPr | DABCO | 82 |
| 14 | THF | TMCTS | IPr | DABCO | 54 |
| 15 | DCE | TMCTS | IPr | DABCO | 10 |
| 16 | Butyronitrile | TMCTS | IPr | DABCO | 72 |
| Entry | Solvent | Time/h | T/℃ | Yield/% |
|---|---|---|---|---|
| 1 | CH3CN | 12 | r.t | n.d. |
| 2 | CH3CN | 12 | 60 | Ttrace |
| 3 | CH3CN | 12 | 80 | 45 |
| 4 | CH3CN | 12 | 100 | 50 |
| 5 | CH3CN | 12 | 120 | 53 |
| 6 | CH3CN | 12 | 140 | 60 |
| 7 | CH3CN | 24 | 140 | Trace |
| 8 | CH3CN | 16 | 140 | 50 |
| 9 | CH3CN | 10 | 140 | 58 |
| 10 | CH3CN | 8 | 140 | 65 |
| 11 | CH3CN | 6 | 140 | 76 |
| 12 | CH3CN | 6 | 160 | 66 |
| 13 | CH3CN | 4 | 140 | 50 |
| Entry | Solvent | Time/h | T/℃ | Yield/% |
|---|---|---|---|---|
| 1 | CH3CN | 12 | r.t | n.d. |
| 2 | CH3CN | 12 | 60 | Ttrace |
| 3 | CH3CN | 12 | 80 | 45 |
| 4 | CH3CN | 12 | 100 | 50 |
| 5 | CH3CN | 12 | 120 | 53 |
| 6 | CH3CN | 12 | 140 | 60 |
| 7 | CH3CN | 24 | 140 | Trace |
| 8 | CH3CN | 16 | 140 | 50 |
| 9 | CH3CN | 10 | 140 | 58 |
| 10 | CH3CN | 8 | 140 | 65 |
| 11 | CH3CN | 6 | 140 | 76 |
| 12 | CH3CN | 6 | 160 | 66 |
| 13 | CH3CN | 4 | 140 | 50 |
| [1] |
Balaban, A. T.; Oniciu, D. C.; Katritzky, A. R. Chem. Rev. 2004, 104, 2777.
|
| [2] |
La Regina, G.; Silvestri, R.; Artico, M.; Lavecchia, A.; Novellino, E.; Befani, O.; Turini, P.; Agostinelli, E. J. Med. Chem. 2007, 50, 922.
|
| [3] |
Pinder, A. R. Nat. Prod. Rep. 1992, 9, 17.
pmid: 1579259 |
| [4] |
Srinivasan, V.; Panneerselvam, M.; Pavithra, N.; Anandan, S.; Sundaravel, K.; Jaccob, M.; Kathiravan, A. J. Photochem. Photobiol. A 2017, 332, 453.
|
| [5] |
Lei, Y.-R.; Xiong, T.-K.; Yu, X.-T.; Huang, X.-B.; Tang, X.-L.; Yi, H.-H.; Zhou, Y.-S.; Zhao, S.-Z.; Sun, L.; Gao, F.-Y. Acta Chim. Sinica 2024, 82, 396. (in Chinese)
|
|
(雷雅茹, 熊廷楷, 于湘涛, 黄秀兵, 唐晓龙, 易红宏, 周远松, 赵顺征, 孙龙, 高凤雨, 化学学报, 2024, 82, 396.)
doi: 10.6023/A23120529 |
|
| [6] |
Cui, G.-Q.; Hu, Y.-Y.; Lou, Y.-J.; Zhou, M.-X.; Li, Y.-M.; Wang, Y.-J.; Jiang, G.-Y.; Xu, C.-M. Acta Chim. Sinica 2023, 81, 1081. (in Chinese)
|
|
(崔国庆, 胡溢玚, 娄颖洁, 周明霞, 李宇明, 王雅君, 姜桂元, 徐春明, 化学学报, 2023, 81, 1081.)
doi: 10.6023/A23040126 |
|
| [7] |
Jiang, Y.-L.; Li, G.-C.; Chen, Q.-S.; Xu, Z.-N.; Lin, S.-S.; Guo, G.-C. Acta Chim. Sinica 2022, 80, 703. (in Chinese)
|
|
(蒋银龙, 李国超, 陈青松, 徐忠宁, 林姗姗, 郭国聪, 化学学报, 2022, 80, 703.)
doi: 10.6023/A22010012 |
|
| [8] |
Song, Y.-P.; Yin, K.; Chen, Y.-J.; Zhao, B.; Zhang, Y.; Zhu, X.-H.; Yuan, D.; Yao, Y.-M. Chin. J. Chem. 2023, 41, 805.
|
| [9] |
Wei, Z.-M.; Liu, Y.-H.; Ding, J.; He, Q.-Y.; Zhang, Q.; Zhai, Y.-M. Chin. J. Chem. 2023, 41, 3553.
|
| [10] |
Liu, J.-Y.; Li, P.-S.; Bi, J.-H.; Wang, Y.; Zhu, Q.-G.; Sun, X.-F.; Zhang, J.-L.; Liu, Z.-M.; Han, B.-X. Chin. J. Chem. 2023, 41, 1443.
|
| [11] |
Hubbard, H.; Lawitz, E. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 9.
|
| [12] |
Popovici-Muller, J.; Lemieux, R. M.; Artin, E.; Saunders, J. O.; Salituro, F. G.; Travins, J.; Cianchetta, G.; Cai, Z. W.; Zhou, D.; Cui, D. W.; Chen, P.; Straley, K.; Tobin, E.; Wang, F.; David, M. D.; Penard-Lacronique, V.; Quivoron, C.; Saada, V.; Botton, S.; Gross, S.; Dang, L.; Yang, H.; Utley, L.; Chen, Y.; Kim, H.; Jin, S. F.; Gu, Z. W.; Yao, G.; Luo, Z. Y.; Lv, X. B.; Fang, C.; Yan, L. P.; Olaharski, A.; Silverman, L.; Biller, S.; Su, S. M.; Yen, K. ACS Med. Chem. Lett. 2018, 9, 300.
doi: 10.1021/acsmedchemlett.7b00421 pmid: 29670690 |
| [13] |
Vangapandu, H. V.; Jain, N.; Gandhi, V. Expert Opin. Invest. Drugs 2017, 26, 625.
|
| [14] |
Sinkule, J. A. Pharmacotherapy 1984, 4, 61.
pmid: 6326063 |
| [15] |
Sarma, M.; Chatterjee, T.; Ghanta, S.; Daset, S. K. J. Org. Chem. 2012, 77, 432.
|
| [16] |
Cui, X.-J.; Dai, X.-C.; Deng, Y.-Q.; Shi, F. Chem.-Eur. J. 2013, 19, 3665.
|
| [17] |
Watanabe, Y.; Shim, S. C.; Uchida, H.; Mitsudo, T.; Takegami, Y. Tetrahedron 1979, 35, 1433.
|
| [18] |
Cano, R.; Ramón, D. J.; Yus, M. J. Org. Chem. 2011, 76, 654.
|
| [19] |
Korbad, B. L.; Lee, S. H. Chem. Commun. 2014, 50, 8985.
|
| [20] |
Tominaga, H. Int. J. Soc. Mater. Eng. Resour. 1995, 3, 33.
|
| [21] |
Liu, Q.; Wu, L.; Jackstell, R.; Beller, M. Nat. Commun. 2015, 6, 5933.
|
| [22] |
Klankermayer, J.; Wesselbaum, S.; Beydoun, K.; Leitner, W. Angew. Chem., Int. Ed. 2016, 55, 7296.
|
| [23] |
Zhang, L.-L.; Han, Z.-B.; Zhang, L.; Li, M.-X.; Ding, K.-L. Chin. J. Org. Chem. 2016, 36, 1824. (in Chinese)
|
|
(张琳莉, 韩召斌, 张磊, 李明星, 丁奎岭, 有机化学, 2016, 36, 1824.)
doi: 10.6023/cjoc201603014 |
|
| [24] |
Tlili, A.; Blondiaux, E.; Frogneux, X.; Cantat, T. Green Chem. 2015, 17, 157.
|
| [25] |
Li, Y.; Cui, X.; Dong, K.; Junge, K.; Beller, M. ACS Catal. 2017, 7, 1077.
|
| [26] |
Cabrero-Antonino, J. R.; Adam, R.; Beller, M. Angew. Chem., Int. Ed. 2019, 58, 12820.
|
| [27] |
Zhang, Y.; Zhang, T.; Das, S. Green Chem. 2020, 22, 1800.
|
| [28] |
Huang, K.; Sun, C.-L.; Shi, Z.-J. Chem. Soc. Rev. 2011, 40, 2435.
doi: 10.1039/c0cs00129e pmid: 21387036 |
| [29] |
Zhang, W.-X.; Liao, P.-Q.; Lin, R.-B.; Wei, Y.-S.; Zeng, M.-H.; Chen, X.-M. Coord. Chem. Rev. 2015, 293-294, 263.
|
| [30] |
Zhang, H.; Sun, H.-J.; Li, X.-Y. Chin. J. Org. Chem. 2016, 36, 2843. (in Chinese)
|
|
(仉花, 孙宏建, 李晓燕, 有机化学, 2016, 36, 2843.)
doi: 10.6023/cjoc201605037 |
|
| [31] |
Zhang, W.-Z.; Zhang, N.; Guo, C.-X.; Lü, X.-B. Chin. J. Org. Chem. 2017, 37, 1309. (in Chinese)
|
|
(张文珍, 张宁, 郭春晓, 吕小兵, 有机化学, 2017, 37, 1309.)
|
|
| [32] |
Chen, J.; McGraw, M.; Chen, E. Y.-X. ChemSusChem. 2019, 12, 4543.
|
| [33] |
Wang, X.; Xia, C.; Wu, L. Green Chem. 2018, 20, 5415.
|
| [34] |
Duan, D.-S.; Ma, Y.; Liu, Y.-B.; Cheng, F.; Zhu, D.-Y.; Wang, S.-H. Chin. J. Org. Chem. 2024, 44, 1675. (in Chinese)
|
|
(段东森, 马媛, 刘宇博, 程富, 朱道勇, 王少华, 有机化学, 2024, 44, 1675.)
doi: 10.6023/cjoc202312018 |
|
| [35] |
Li, B.; Sortais, J.-B.; Darcel, C. RSC Adv. 2016, 6, 57603.
|
| [36] |
Addis, D.; Das, S.; Junge, K.; Beller, M. Angew. Chem., Int. Ed. 2011, 50, 6004.
|
| [37] |
Ostapowicz, T. G.; Schmitz, M.; Krystof, M.; Klankermayer, J.; Leitner, W. Angew. Chem., Int. Ed. 2013, 52, 12119.
|
| [38] |
Wu, L.; Liu, Q.; Fleischer, I.; Jackstell, R.; Beller, M. Nat. Commun. 2014, 5, 3091.
|
| [39] |
Ren, X.; Zheng, Z.; Zhang, L.; Wang, Z.; Xia, C.; Ding, K. Angew. Chem., Int. Ed. 2017, 56, 310.
|
| [40] |
Guo, Z.; Yang, B.; Jia, J.; Wei, X. Asian. J. Org. Chem. 2023, 12, e202300097.
|
| [41] |
Thenert, K.; Beydoun, K.; Wiesenthal, J.; Leitner, W.; Klankermayer, J. Angew. Chem., Int. Ed. 2016, 55, 12266.
|
| [42] |
Jin, G.; Werncke, C. G.; Escudié, Y.; Sabo-Etienne, S.; Bontemps, S. J. Am. Chem. Soc. 2015, 137, 9563.
|
| [43] |
Frogneux, X.; Blondiaux, E.; Thuéry, P.; Cantat, T. ACS Catal. 2015, 5, 3983.
|
| [44] |
Liu, M.; Qin, T.; Zhang, Q.; Fang, C.; Fu, Y.; Lin, B.-L. Sci. China: Chem. 2015, 58, 1524.
|
| [45] |
Yu, Z.; Li, Z. Y.; Zhang, L. L.; Zhu, K. X.; Wu, H. G.; Li, H.; Yang, S. Green Chem. 2021, 23, 5759.
|
| [46] |
Zhu, D.-Y.; Fang, L.; Han, H.; Wang, Y.; Xia, J.-B. Org. Lett. 2017, 19, 4259.
|
| [47] |
Li, W.; Chen, J.; Zhu, D.; Xia, J. Chin. J. Chem. 2021, 39, 614.
|
| [48] |
Miles, D. H.; Huang, B.-S. J. Org. Chem. 1976, 41, 208.
|
| [49] |
Nusse, R.; Clevers, H. Cell 2017, 169, 985.
doi: S0092-8674(17)30547-0 pmid: 28575679 |
| [50] |
Kakugawa, S.; Langton, P.; Zebisch, M.; Howell, S.; Chang, T.; Liu, Y.; Feizi, T.; Bineva, G.; O'Reilly, N.; Snijders, A.; Jones, E.; Vincent, J. Nature 2015, 519, 187.
|
| [51] |
Zhang, X.; Cheong, S.; Amado, N.; Reis, A.; MacDonald, B.; Zebisch, M.; Jones, E.; Abreu, J.; He, X. Dev. Cell 2015, 32, 719.
|
| [52] |
Mahy, W.; Patel, M.; Steadman, D.; Woodward, H. L.; Atkinson, B. N.; Svensson, F.; Willis, N. J.; Flint, A.; Papatheodorou, D.; Zhao, Y.; Vecchia, L.; Ruza, R. R.; Hillier, J.; Frew, S.; Monaghan, A.; Costa, A.; Bictash, M.; Walter, M. W.; Jones, E. Y.; Fish, P. V. J. Med. Chem. 2020, 63, 9464.
|
| [53] |
Zhu, D.-Y.; Wang, S.-H. CN 202311774883, 7, 2023.
|
| [1] | 高晋彬, 陆颖琪, 张辉, 高利柱, 熊兴泉. 生物质基催化剂在CO2化学转化中的应用[J]. 有机化学, 2024, 44(9): 2732-2741. |
| [2] | 李建文, 王涛, 陶晟, 陈飞, 李敏, 刘宁. SBA-15负载的N-杂环卡宾-吡啶钼配合物在二氧化碳转化制备环状碳酸酯中的应用[J]. 有机化学, 2024, 44(10): 3213-3222. |
| [3] | 何君, 王红星, 余成龙, 张艳茹, 王莹, 王燕燕, 张龙博, 郭佳, 钱庆利, 韩布兴. 纳米花状Ir/MoS2催化剂用于CO2加氢高选择性制备甲酸盐[J]. 有机化学, 2024, 44(10): 3223-3232. |
| [4] | 陈学伟, 于方彩, 田传洪. 1,1'-亚甲基二咪唑鎓多氢键供体催化剂促进常压下CO2与环氧化物的环加成反应[J]. 有机化学, 2024, 44(10): 3198-3205. |
| [5] | 黄文翰, 姜山, 李慧, 赵凤玉, 程海洋. 二氧化碳基可再加工微交联热固性聚脲的合成[J]. 有机化学, 2024, 44(10): 3178-3184. |
| [6] | 王艳伟, 陈薇薇, 袁丹, 张勇, 姚英明. 杂核金属配合物催化CO2和环氧烷反应的研究进展[J]. 有机化学, 2024, 44(10): 3063-3076. |
| [7] | 王凯悦, 许明诺, 李博, 伍广朋. 双功能硫脲催化剂在“一锅法”制备多肽和环状碳酸酯反应中的应用[J]. 有机化学, 2024, 44(10): 3206-3212. |
| [8] | 李文珂, 孙北奇, 张雷, 莫凡洋. 基于自由基机理光催化羧基化反应研究进展[J]. 有机化学, 2024, 44(10): 2961-2996. |
| [9] | 肖丽娟, 张艳平, 洪缪. 路易斯酸碱对在材料化学应用中的研究进展[J]. 有机化学, 2023, 43(3): 949-960. |
| [10] | 刘桂杰, 付正强, 陈飞, 徐彩霞, 李敏, 刘宁. N-杂环卡宾-吡啶锰配合物/四丁基碘化铵催化CO2和环氧化物合成环状碳酸酯[J]. 有机化学, 2023, 43(2): 629-635. |
| [11] | 窦谦, 汪太民, 李嗣锋, 房丽晶, 翟宏斌, 程斌. 光催化CO2参与的σ键断裂羧基化反应研究进展[J]. 有机化学, 2022, 42(12): 4257-4274. |
| [12] | 李勇, 王征, 刘庆彬. CO2均相催化氢化研究进展[J]. 有机化学, 2017, 37(8): 1978-1990. |
| [13] | 仉花, 孙宏建, 李晓燕. 过渡金属氢化物在CO2活化和功能化反应中的应用[J]. 有机化学, 2016, 36(12): 2843-2857. |
| [14] | 朱庆, 王露, 夏春谷, 刘超. 过渡金属催化CO2与C-H键直接羧化反应的研究进展[J]. 有机化学, 2016, 36(12): 2813-2821. |
| [15] | 林美玉; 王 欢; 张爱健 ; 张贵荣 ; 陆嘉星*. 电羧化苯乙烯基苯基酮合成2,4-二苯基-4-丁酮酸[J]. 有机化学, 2008, 28(9): 1572-1577. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||