

选择性C—H键氯化反应进展
收稿日期: 2024-06-21
修回日期: 2024-08-26
网络出版日期: 2024-09-27
基金资助
河南省自然科学基金(222300420056); 河南省自然科学基金(222300420204)
Recent Progress in Selective C—H Chlorination
Received date: 2024-06-21
Revised date: 2024-08-26
Online published: 2024-09-27
Supported by
Natural Science Foundation of Henan Province(222300420056); Natural Science Foundation of Henan Province(222300420204)
王娟娟 , 史妍 , 田英贤 , 刘丙贤 . 选择性C—H键氯化反应进展[J]. 有机化学, 2025 , 45(2) : 574 -591 . DOI: 10.6023/cjoc202406030
The importance of organic chlorides in pharmaceutical, material, and synthetic chemistry has made the constru- ction of C—Cl bonds a significant focus in organic chemistry. The cleavage and functionalization of C—H bonds with high atomic efficiency, leading to direct conversion into desired functional groups, is seen as a promising approach for creating new substances. Recent advancements in C—H bond activation/tranformation have led to the development of C—H chlorination reactions as viable alternatives to traditional halogenation methods. This review provides an overview of the progress made in C—H bond chlorination reactions over the last decade.
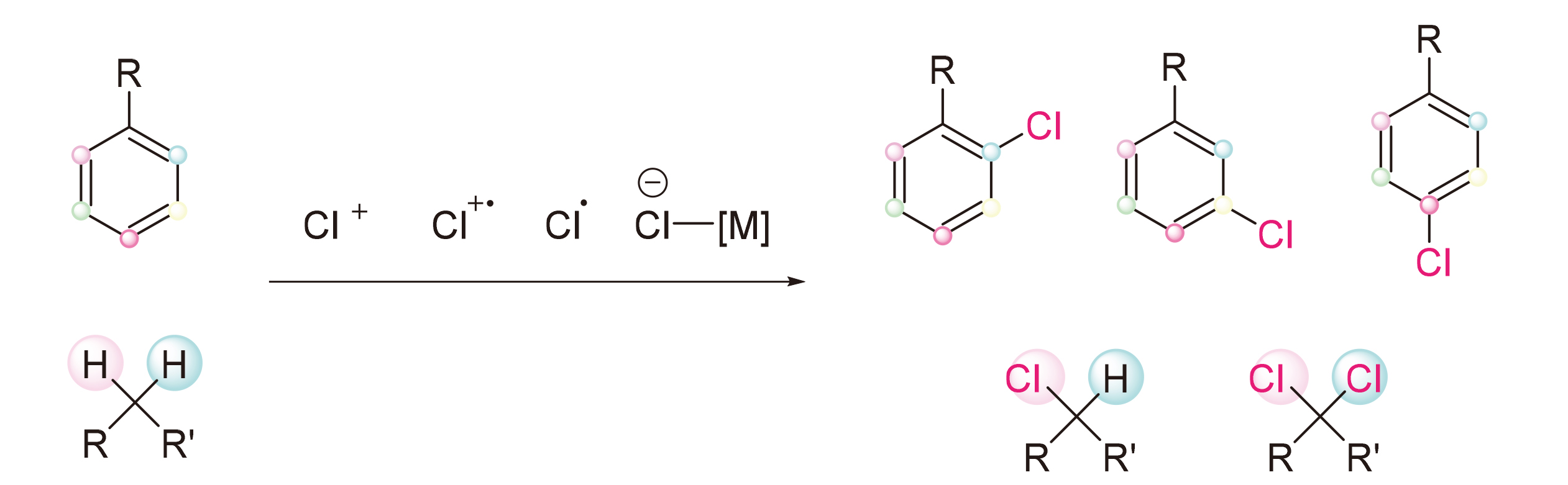
Key words: chlorination; C—H bonds; selective; umpolung
| [1] | Smith, B. R.; Eastman, C. M.; Njardarson, J. T. J. Med. Chem. 2014, 57, 9764. |
| [2] | Tang, M. L.; Bao, Z. Chem. Mater. 2010, 23, 446. |
| [3] | Nishii, Y.; Ikeda, M.; Hayashi, Y.; Kawauchi, S.; Miura, M. J. Am. Chem. Soc. 2020, 142, 1621. |
| [4] | Lin, R.; Amrute, A. P.; Perez-Ramirez, J. Chem. Rev. 2017, 117, 4182. |
| [5] | Petrone, D. A.; Ye, J.; Lautens, M. Chem. Rev. 2016, 116, 8003. |
| [6] | Ding, L.; Tang, J.; Cui, M.; Bo, C.; Chen, X.; Qiao, X. Ind. Eng. Chem. Res. 2011, 50, 11143. |
| [7] | Cambeiro, X. C.; Ahlsten, N.; Larrosa, I. J. Am. Chem. Soc. 2015, 137, 15636. |
| [8] | Liu, S.; Zhang, Q.; Li, H.; Yang, Y.; Tian, X.; Whiting, A. Chem.- Eur. J. 2015, 21, 9671. |
| [9] | Goldsmith, C. R.; Coates, C. M.; Hagan, K.; Mitchell, C. A. J. Mol. Catal. A: Chem. 2011, 335, 24. |
| [10] | Pu, M.; He, F. Acta Chim. Sinica 2023, 81, 1541 (in Chinese). |
| [10] | (蒲明瑞, 何凤, 化学学报, 2023, 81, 1541.) |
| [11] | Iwasa, E.; Hamashima, Y.; Fujishiro, S.; Higuchi, E.; Ito, A.; Yoshida, M.; Sodeoka, M. J. Am. Chem. Soc. 2010, 132, 4078. |
| [12] | Ohkita, T.; Tsuchiya, Y.; Togo, H. Tetrahedron 2008, 64, 7247. |
| [13] | Tay, D. W. P.; Nobbs, J. D.; Romain, C.; White, A. J. P.; Aitipamula, S.; van Meurs, M.; Britovsek, G. J. P. ACS Catal. 2019, 10, 663. |
| [14] | Kumaran, S.; Parthasarathy, K. J. Org. Chem. 2021, 86, 7987. |
| [15] | Wang, Q.; Zhang, W. W.; Song, H.; Wang, J.; Zheng, C.; Gu, Q.; You, S. L. J. Am. Chem. Soc. 2020, 142, 15678. |
| [16] | Yi, J.; Chen, M. Acta Chim. Sinica 2024, 82, 126 (in Chinese). |
| [16] | (易敬霖, 陈茂, 化学学报, 2024, 82, 126.) |
| [17] | Monnier, F.; Taillefer, M. Angew. Chem., Int. Ed. 2009, 48, 6954. |
| [18] | Cui, C.; Dai, M. Chin. J. Chem. 2023, 41, 3019. |
| [19] | Su, J.; Li, C.; Hu, X.; Guo, Y.; Song, Q. Angew. Chem., Int. Ed. 2022, 61, e202212740. |
| [20] | Bhoyare, V. W.; Sosa Carrizo, E. D.; Chintawar, C. C.; Gandon, V.; Patil, N. T. J. Am. Chem. Soc. 2023, 145, 8810. |
| [21] | Helbert, H.; Visser, P.; Hermens, J. G. H.; Buter, J.; Feringa, B. L. Nat. Catal. 2020, 3, 664. |
| [22] | Forero-Cortés, P. A.; Haydl, A. M. Org. Process Res. Dev. 2019, 23, 1478. |
| [23] | Liu, Y.; Fang, Y.; Zhang, L.; Jin, X.; Li, R.; Zhu, S.; Gao, H.; Fang, J.; Xia, Q. Chin. J. Org. Chem. 2014, 34, 1523 (in Chinese). |
| [23] | (刘雨燕, 方烨汶, 张莉, 金小平, 李瑞丰, 朱帅汝, 高浩其, 房江华, 夏勤波, 有机化学, 2014, 34, 1523.) |
| [24] | Ziegler, D. S.; Wei, B.; Knochel, P. Chem. Eur. J. 2019, 25, 2695. |
| [25] | Das, R.; Kapur, M. Asian J. Org. Chem. 2018, 7, 1524. |
| [26] | Chen, J.; Lin, J. H.; Xiao, J. C. Org. Lett. 2018, 20, 3061. |
| [27] | Samanta, R. C.; Yamamoto, H. Chem.-Eur. J. 2015, 21, 11976. |
| [28] | Maddox, S. M.; Nalbandian, C. J.; Smith, D. E.; Gustafson, J. L. Org. Lett. 2015, 17, 1042. |
| [29] | Werf, A.; Selander, N. Org. Lett. 2015, 17, 6210. |
| [30] | Maddox, S. M.; Dinh, A. N.; Armenta, F.; Um, J.; Gustafson, J. L. Org. Lett. 2016, 18, 5476. |
| [31] | Xiong, X.; Yeung, Y.-Y. ACS Catal. 2018, 8, 4033. |
| [32] | Song, S.; Li, X.; Wei, J.; Wang, W.; Zhang, Y.; Ai, L.; Zhu, Y.; Shi, X.; Zhang, X.; Jiao, N. Nat. Catal. 2019, 3, 107. |
| [33] | Li, G.; Zhu, B.; Ma, X.; Jia, C.; Lv, X.; Wang, J.; Zhao, F.; Lv, Y.; Yang, S. Org. Lett. 2017, 19, 5166. |
| [34] | Fan, Z.; Lu, H.; Cheng, Z.; Zhang, A. Chem Commun. 2018, 54, 6008. |
| [35] | Zhang, H.; Xu, M.; Liu, N.; Yang, F. ChemistrySelect 2021, 6, 2319. |
| [36] | Fosu, S. C.; Hambira, C. M.; Chen, A. D.; Fuchs, J. R.; Nagib, D. A. Chem 2019, 5, 417. |
| [37] | Segura-Quezada, A.; Satkar, Y.; Patil, D.; Mali, N.; Wrobel, K.; González, G.; Zárraga, R.; Ortiz-Alvarado, R.; Solorio-Alvarado, C. R. Tetrahedron Lett. 2019, 60, 1551. |
| [38] | Granados, A.; Jia, Z.; del Olmo, M.; Vallribera, A. Eur. J. Org. Chem. 2019, 2019, 2812. |
| [39] | Hering, T.; K?nig, B. Tetrahedron 2016, 72, 7821. |
| [40] | Düsel, S. J. S.; K?nig, B. Eur. J. Org. Chem. 2019, 2020, 1491. |
| [41] | Rogers, D. A.; Gallegos, J. M.; Hopkins, M. D.; Lignieres, A. A.; Pitzel, A. K.; Lamar, A. A. Tetrahedron 2019, 75, 130498. |
| [42] | Zhang, L.; Hu, X. Chem. Sci. 2017, 8, 7009. |
| [43] | Xie, W. C.; Wang, M.; Yang, S.; Chen, Y. D.; Feng, J.; Huang, Y. T. Org. Biomol. Chem. 2022, 20, 5319. |
| [44] | Harnedy, J.; Hareram, M. D.; Tizzard, G. J.; Coles, S. J.; Morrill, L. C. Chem. Commun. 2021, 57, 12643. |
| [45] | Liu, T.; Lu, H. K.; Shi, Z.; Yan, H.; Li, Z.; Ye, K. Y. Eur. J. Org. Chem. 2024, 27, e202300880. |
| [46] | Fahey, D. R. Chem. Commun. 1970, 7, 417a. |
| [47] | Du, B.; Jiang, X.; Sun, P. J. Org. Chem. 2013, 78, 2786. |
| [48] | Mo, S.; Zhu, Y.; Shen, Z. Org. Biomol. Chem. 2013, 11, 2756. |
| [49] | Qian, G.; Hong, X.; Liu, B.; Mao, H.; Xu, B. Org. Lett. 2014, 16, 5294. |
| [50] | Li, B.; Liu, B.; Shi, B. F. Chem. Commun. 2015, 51, 5093. |
| [51] | Zhan, B. B.; Liu, Y. H.; Hu, F.; Shi, B. F. Chem. Commun. 2016, 52, 4934. |
| [52] | Kathiravan, S.; Nicholls, I. A. Chem.-Eur. J. 2017, 23, 7031. |
| [53] | Zhu, Y.; Huang, J.; Yang, X. Chin. J. Org. Chem. 2019, 39, 1665 (in Chinese). |
| [53] | (朱晔, 黄金文, 杨先金, 有机化学, 2019, 39, 1665.) |
| [54] | Shi, H.; Wang, P.; Suzuki, S.; Farmer, M. E.; Yu, J. Q. J. Am. Chem. Soc. 2016, 138, 14876. |
| [55] | Wang, Y.; Li, G. X.; Yang, G.; He, G.; Chen, G. Chem. Sci. 2016, 7, 2679. |
| [56] | Zhao, M.; Lu, W. Org Lett. 2017, 19, 4560. |
| [57] | Xiang, M.; Zhou, C.; Yang, X. L.; Chen, B.; Tung, C. H.; Wu, L. Z. J. Org. Chem. 2020, 85, 9080. |
| [58] | He, Q.; Cao, Z.; Zhang, Y.; Chen, G.; Wang, Y. Adv. Synth. Catal. 2023, 365, 2711. |
| [59] | McMillan, A. J.; Sienkowska, M.; Di Lorenzo, P.; Gransbury, G. K.; Chilton, N. F.; Salamone, M.; Ruffoni, A.; Bietti, M.; Leonori, D. Angew. Chem., Int. Ed. 2021, 60, 7132. |
| [60] | Liu, R. Z.; Li, J.; Sun, J.; Liu, X. G.; Qu, S.; Li, P.; Zhang, B. Angew. Chem., Int. Ed. 2020, 59, 4428. |
| [61] | Zhu, Y.; Shi, J.; Yu, W. Org. Lett. 2020, 22, 8899. |
| [62] | Herron, A. N.; Liu, D.; Xia, G.; Yu, J. Q. J. Am. Chem. Soc. 2020, 142, 2766. |
| [63] | He, Y.; Tian, C.; An, G.; Li, G. Chin. Chem. Lett. 2024, 35, 108546. |
| [64] | Short, M. A.; Blackburn, J. M.; Roizen, J. L. Angew. Chem., Int. Ed. 2018, 57, 296. |
| [65] | Zhao, J.; Zhang, J.; Fang, P.; Wu, J.; Wang, F.; Liu, Z.-Q. Green Chem. 2024, 26, 507. |
| [66] | Ozawa, J.; Kanai, M. Org. Lett. 2017, 19, 1430. |
| [67] | Li, G.; Dilger, A. K.; Cheng, P. T.; Ewing, W. R.; Groves, J. T. Angew. Chem., Int. Ed. 2018, 57, 1251. |
| [68] | Jin, J.; Zhao, Y.; Kyne, S. H.; Farshadfar, K.; Ariafard, A.; Chan, P. W. H. Nat. Commun. 2021, 12, 4065. |
| [69] | Lopez, M. A.; Buss, J. A.; Stahl, S. S. Org. Lett. 2022, 24, 597. |
| [70] | Stowers, K. J.; Kubota, A.; Sanford, M. S. Chem. Sci. 2012, 3, 3192. |
| [71] | Li, B.; Wang, S.-Q.; Liu, B.; Shi, B.-F. Org. Lett. 2015, 17, 1200. |
| [72] | Liu, W.; Tan, L.; Zhou, P.; Chen, C.; Zhang, Q. Synthesis 2013, 45, 2600. |
| [73] | Su, Y.; Shi, Y.; Chang, B.; Wu, L.; Chong, S.; Zhang, W.; Huang, D.; Wang, K.; Hu, Y. Chin. J. Org. Chem. 2018, 38, 1454 (in Chinese). |
| [73] | (苏瀛鹏, 石娅, 常兵兵, 吴丽丽, 种思颖, 张为钢, 黄丹凤, 王克虎, 胡雨来, 有机化学, 2018, 38, 1454.) |
| [74] | Rit, R. K.; Yadav, M. R.; Ghosh, K.; Shankar, M.; Sahoo, A. K. Org. Lett. 2014, 16, 5258. |
| [75] | Xiong, H. Y.; Cahard, D.; Pannecoucke, X.; Besset, T. Eur. J. Org. Chem. 2016, 2016, 3625. |
| [76] | Yang, X.; Sun, Y.; Sun, T. Y.; Rao, Y. Chem. Commun. 2016, 52, 6423. |
/
| 〈 |
|
〉 |