有机化学 ›› 2025, Vol. 45 ›› Issue (2): 574-591.DOI: 10.6023/cjoc202406030 上一篇 下一篇
综述与进展
收稿日期:2024-06-21
修回日期:2024-08-26
发布日期:2024-09-26
作者简介:基金资助:
Juanjuan Wang, Yan Shi, Yingxian Tian, Bingxian Liu( )
)
Received:2024-06-21
Revised:2024-08-26
Published:2024-09-26
Contact:
*E-mail: liubingxian@htu.edu.cn
About author:Supported by:文章分享
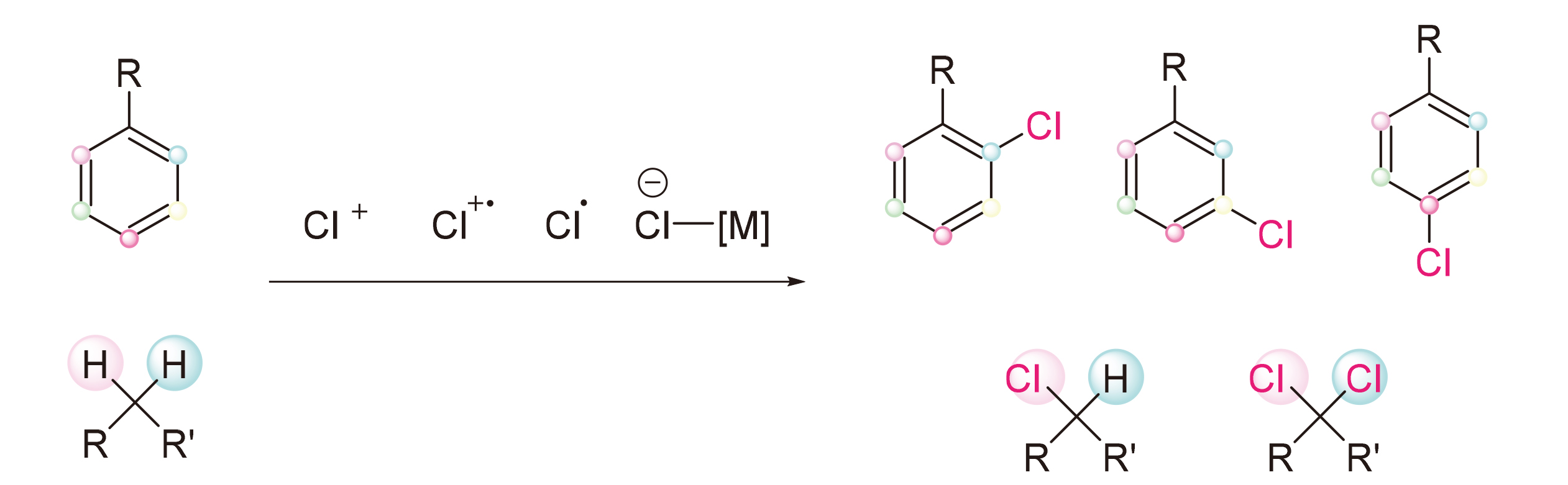
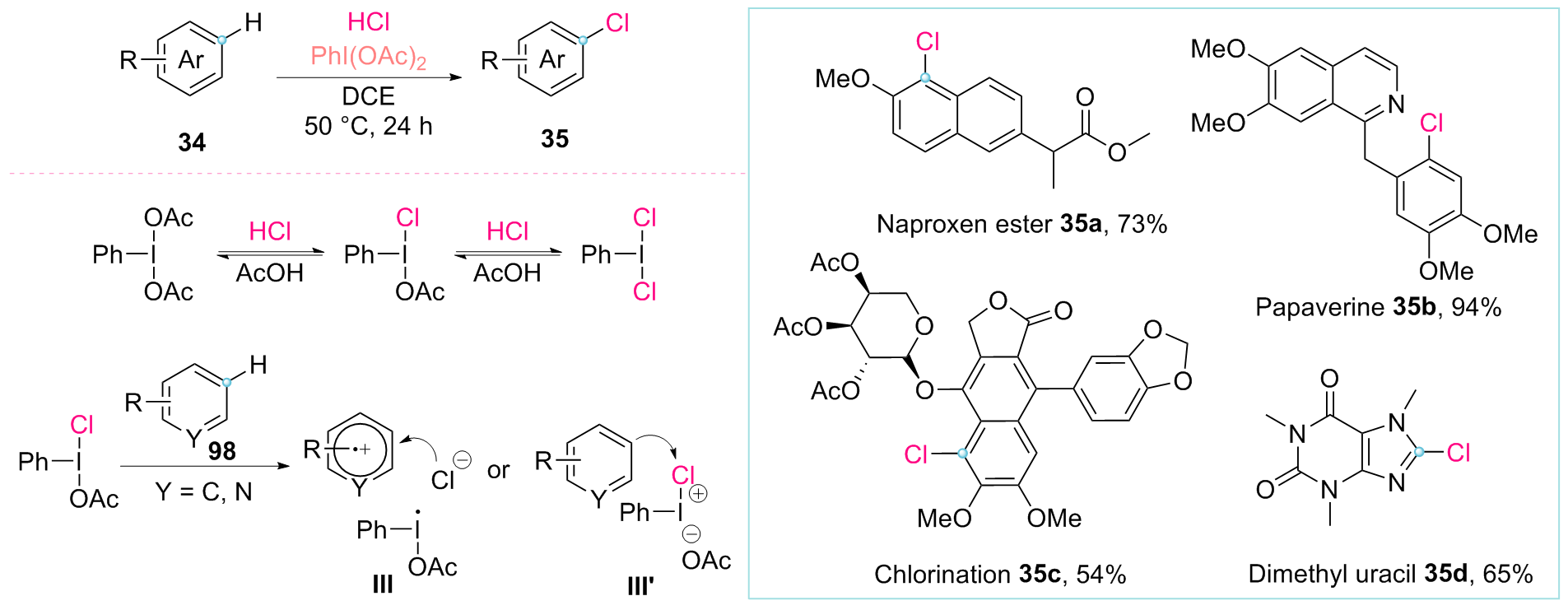
由于有机氯化物在药物化学、材料化学以及合成化学领域的重要作用, 碳氯键的构建一直是有机化学方向热点研究领域. 由于通过碳氢键断裂并直接转化为目标官能团具有高度的原子经济性, 其被认为是新一代极具潜力的物质构建和转化途径. 近年来, 随着碳氢键活化转化研究的发展, 一些C—H键氯化反应已被开发为传统卤化方法的实用替代方案, 此综述对近十年C—H键氯化反应的发展进行了归纳总结.
王娟娟, 史妍, 田英贤, 刘丙贤. 选择性C—H键氯化反应进展[J]. 有机化学, 2025, 45(2): 574-591.
Juanjuan Wang, Yan Shi, Yingxian Tian, Bingxian Liu. Recent Progress in Selective C—H Chlorination[J]. Chinese Journal of Organic Chemistry, 2025, 45(2): 574-591.


| [1] |
Smith, B. R.; Eastman, C. M.; Njardarson, J. T. J. Med. Chem. 2014, 57, 9764.
|
| [2] |
Tang, M. L.; Bao, Z. Chem. Mater. 2010, 23, 446.
|
| [3] |
Nishii, Y.; Ikeda, M.; Hayashi, Y.; Kawauchi, S.; Miura, M. J. Am. Chem. Soc. 2020, 142, 1621.
doi: 10.1021/jacs.9b12672 pmid: 31868360 |
| [4] |
Lin, R.; Amrute, A. P.; Perez-Ramirez, J. Chem. Rev. 2017, 117, 4182.
|
| [5] |
Petrone, D. A.; Ye, J.; Lautens, M. Chem. Rev. 2016, 116, 8003.
doi: 10.1021/acs.chemrev.6b00089 pmid: 27341176 |
| [6] |
Ding, L.; Tang, J.; Cui, M.; Bo, C.; Chen, X.; Qiao, X. Ind. Eng. Chem. Res. 2011, 50, 11143.
|
| [7] |
Cambeiro, X. C.; Ahlsten, N.; Larrosa, I. J. Am. Chem. Soc. 2015, 137, 15636.
doi: 10.1021/jacs.5b10593 pmid: 26645996 |
| [8] |
Liu, S.; Zhang, Q.; Li, H.; Yang, Y.; Tian, X.; Whiting, A. Chem.- Eur. J. 2015, 21, 9671.
|
| [9] |
Goldsmith, C. R.; Coates, C. M.; Hagan, K.; Mitchell, C. A. J. Mol. Catal. A: Chem. 2011, 335, 24.
|
| [10] |
Pu, M.; He, F. Acta Chim. Sinica 2023, 81, 1541 (in Chinese).
|
|
(蒲明瑞, 何凤, 化学学报, 2023, 81, 1541.)
doi: 10.6023/A23050245 |
|
| [11] |
Iwasa, E.; Hamashima, Y.; Fujishiro, S.; Higuchi, E.; Ito, A.; Yoshida, M.; Sodeoka, M. J. Am. Chem. Soc. 2010, 132, 4078.
|
| [12] |
Ohkita, T.; Tsuchiya, Y.; Togo, H. Tetrahedron 2008, 64, 7247.
|
| [13] |
Tay, D. W. P.; Nobbs, J. D.; Romain, C.; White, A. J. P.; Aitipamula, S.; van Meurs, M.; Britovsek, G. J. P. ACS Catal. 2019, 10, 663.
|
| [14] |
Kumaran, S.; Parthasarathy, K. J. Org. Chem. 2021, 86, 7987.
|
| [15] |
Wang, Q.; Zhang, W. W.; Song, H.; Wang, J.; Zheng, C.; Gu, Q.; You, S. L. J. Am. Chem. Soc. 2020, 142, 15678.
|
| [16] |
Yi, J.; Chen, M. Acta Chim. Sinica 2024, 82, 126 (in Chinese).
|
|
(易敬霖, 陈茂, 化学学报, 2024, 82, 126.)
doi: 10.6023/A23080387 |
|
| [17] |
Monnier, F.; Taillefer, M. Angew. Chem., Int. Ed. 2009, 48, 6954.
|
| [18] |
Cui, C.; Dai, M. Chin. J. Chem. 2023, 41, 3019.
|
| [19] |
Su, J.; Li, C.; Hu, X.; Guo, Y.; Song, Q. Angew. Chem., Int. Ed. 2022, 61, e202212740.
|
| [20] |
Bhoyare, V. W.; Sosa Carrizo, E. D.; Chintawar, C. C.; Gandon, V.; Patil, N. T. J. Am. Chem. Soc. 2023, 145, 8810.
|
| [21] |
Helbert, H.; Visser, P.; Hermens, J. G. H.; Buter, J.; Feringa, B. L. Nat. Catal. 2020, 3, 664.
|
| [22] |
Forero-Cortés, P. A.; Haydl, A. M. Org. Process Res. Dev. 2019, 23, 1478.
doi: 10.1021/acs.oprd.9b00161 |
| [23] |
Liu, Y.; Fang, Y.; Zhang, L.; Jin, X.; Li, R.; Zhu, S.; Gao, H.; Fang, J.; Xia, Q. Chin. J. Org. Chem. 2014, 34, 1523 (in Chinese).
|
|
(刘雨燕, 方烨汶, 张莉, 金小平, 李瑞丰, 朱帅汝, 高浩其, 房江华, 夏勤波, 有机化学, 2014, 34, 1523.)
doi: 10.6023/cjoc201403003 |
|
| [24] |
Ziegler, D. S.; Wei, B.; Knochel, P. Chem. Eur. J. 2019, 25, 2695.
|
| [25] |
Das, R.; Kapur, M. Asian J. Org. Chem. 2018, 7, 1524.
|
| [26] |
Chen, J.; Lin, J. H.; Xiao, J. C. Org. Lett. 2018, 20, 3061.
doi: 10.1021/acs.orglett.8b01058 pmid: 29741387 |
| [27] |
Samanta, R. C.; Yamamoto, H. Chem.-Eur. J. 2015, 21, 11976.
doi: 10.1002/chem.201502234 pmid: 26185028 |
| [28] |
Maddox, S. M.; Nalbandian, C. J.; Smith, D. E.; Gustafson, J. L. Org. Lett. 2015, 17, 1042.
doi: 10.1021/acs.orglett.5b00186 pmid: 25671756 |
| [29] |
Werf, A.; Selander, N. Org. Lett. 2015, 17, 6210.
|
| [30] |
Maddox, S. M.; Dinh, A. N.; Armenta, F.; Um, J.; Gustafson, J. L. Org. Lett. 2016, 18, 5476.
pmid: 27754679 |
| [31] |
Xiong, X.; Yeung, Y.-Y. ACS Catal. 2018, 8, 4033.
|
| [32] |
Song, S.; Li, X.; Wei, J.; Wang, W.; Zhang, Y.; Ai, L.; Zhu, Y.; Shi, X.; Zhang, X.; Jiao, N. Nat. Catal. 2019, 3, 107.
|
| [33] |
Li, G.; Zhu, B.; Ma, X.; Jia, C.; Lv, X.; Wang, J.; Zhao, F.; Lv, Y.; Yang, S. Org. Lett. 2017, 19, 5166.
|
| [34] |
Fan, Z.; Lu, H.; Cheng, Z.; Zhang, A. Chem Commun. 2018, 54, 6008.
|
| [35] |
Zhang, H.; Xu, M.; Liu, N.; Yang, F. ChemistrySelect 2021, 6, 2319.
doi: 10.1002/slct.202100030 |
| [36] |
Fosu, S. C.; Hambira, C. M.; Chen, A. D.; Fuchs, J. R.; Nagib, D. A. Chem 2019, 5, 417.
|
| [37] |
Segura-Quezada, A.; Satkar, Y.; Patil, D.; Mali, N.; Wrobel, K.; González, G.; Zárraga, R.; Ortiz-Alvarado, R.; Solorio-Alvarado, C. R. Tetrahedron Lett. 2019, 60, 1551.
doi: 10.1016/j.tetlet.2019.05.019 |
| [38] |
Granados, A.; Jia, Z.; del Olmo, M.; Vallribera, A. Eur. J. Org. Chem. 2019, 2019, 2812.
doi: 10.1002/ejoc.201900237 |
| [39] |
Hering, T.; König, B. Tetrahedron 2016, 72, 7821.
|
| [40] |
Düsel, S. J. S.; König, B. Eur. J. Org. Chem. 2019, 2020, 1491.
|
| [41] |
Rogers, D. A.; Gallegos, J. M.; Hopkins, M. D.; Lignieres, A. A.; Pitzel, A. K.; Lamar, A. A. Tetrahedron 2019, 75, 130498.
|
| [42] |
Zhang, L.; Hu, X. Chem. Sci. 2017, 8, 7009.
doi: 10.1039/c7sc03010j pmid: 29147528 |
| [43] |
Xie, W. C.; Wang, M.; Yang, S.; Chen, Y. D.; Feng, J.; Huang, Y. T. Org. Biomol. Chem. 2022, 20, 5319.
|
| [44] |
Harnedy, J.; Hareram, M. D.; Tizzard, G. J.; Coles, S. J.; Morrill, L. C. Chem. Commun. 2021, 57, 12643.
|
| [45] |
Liu, T.; Lu, H. K.; Shi, Z.; Yan, H.; Li, Z.; Ye, K. Y. Eur. J. Org. Chem. 2024, 27, e202300880.
|
| [46] |
Fahey, D. R. Chem. Commun. 1970, 7, 417a.
|
| [47] |
Du, B.; Jiang, X.; Sun, P. J. Org. Chem. 2013, 78, 2786.
|
| [48] |
Mo, S.; Zhu, Y.; Shen, Z. Org. Biomol. Chem. 2013, 11, 2756.
|
| [49] |
Qian, G.; Hong, X.; Liu, B.; Mao, H.; Xu, B. Org. Lett. 2014, 16, 5294.
|
| [50] |
Li, B.; Liu, B.; Shi, B. F. Chem. Commun. 2015, 51, 5093.
|
| [51] |
Zhan, B. B.; Liu, Y. H.; Hu, F.; Shi, B. F. Chem. Commun. 2016, 52, 4934.
|
| [52] |
Kathiravan, S.; Nicholls, I. A. Chem.-Eur. J. 2017, 23, 7031.
doi: 10.1002/chem.201700280 pmid: 28221698 |
| [53] |
Zhu, Y.; Huang, J.; Yang, X. Chin. J. Org. Chem. 2019, 39, 1665 (in Chinese).
|
|
(朱晔, 黄金文, 杨先金, 有机化学, 2019, 39, 1665.)
doi: 10.6023/cjoc201903037 |
|
| [54] |
Shi, H.; Wang, P.; Suzuki, S.; Farmer, M. E.; Yu, J. Q. J. Am. Chem. Soc. 2016, 138, 14876.
|
| [55] |
Wang, Y.; Li, G. X.; Yang, G.; He, G.; Chen, G. Chem. Sci. 2016, 7, 2679.
|
| [56] |
Zhao, M.; Lu, W. Org Lett. 2017, 19, 4560.
|
| [57] |
Xiang, M.; Zhou, C.; Yang, X. L.; Chen, B.; Tung, C. H.; Wu, L. Z. J. Org. Chem. 2020, 85, 9080.
doi: 10.1021/acs.joc.0c01000 pmid: 32434320 |
| [58] |
He, Q.; Cao, Z.; Zhang, Y.; Chen, G.; Wang, Y. Adv. Synth. Catal. 2023, 365, 2711.
|
| [59] |
McMillan, A. J.; Sienkowska, M.; Di Lorenzo, P.; Gransbury, G. K.; Chilton, N. F.; Salamone, M.; Ruffoni, A.; Bietti, M.; Leonori, D. Angew. Chem., Int. Ed. 2021, 60, 7132.
|
| [60] |
Liu, R. Z.; Li, J.; Sun, J.; Liu, X. G.; Qu, S.; Li, P.; Zhang, B. Angew. Chem., Int. Ed. 2020, 59, 4428.
|
| [61] |
Zhu, Y.; Shi, J.; Yu, W. Org. Lett. 2020, 22, 8899.
|
| [62] |
Herron, A. N.; Liu, D.; Xia, G.; Yu, J. Q. J. Am. Chem. Soc. 2020, 142, 2766.
|
| [63] |
He, Y.; Tian, C.; An, G.; Li, G. Chin. Chem. Lett. 2024, 35, 108546.
|
| [64] |
Short, M. A.; Blackburn, J. M.; Roizen, J. L. Angew. Chem., Int. Ed. 2018, 57, 296.
|
| [65] |
Zhao, J.; Zhang, J.; Fang, P.; Wu, J.; Wang, F.; Liu, Z.-Q. Green Chem. 2024, 26, 507.
|
| [66] |
Ozawa, J.; Kanai, M. Org. Lett. 2017, 19, 1430.
doi: 10.1021/acs.orglett.7b00367 pmid: 28256138 |
| [67] |
Li, G.; Dilger, A. K.; Cheng, P. T.; Ewing, W. R.; Groves, J. T. Angew. Chem., Int. Ed. 2018, 57, 1251.
|
| [68] |
Jin, J.; Zhao, Y.; Kyne, S. H.; Farshadfar, K.; Ariafard, A.; Chan, P. W. H. Nat. Commun. 2021, 12, 4065.
|
| [69] |
Lopez, M. A.; Buss, J. A.; Stahl, S. S. Org. Lett. 2022, 24, 597.
|
| [70] |
Stowers, K. J.; Kubota, A.; Sanford, M. S. Chem. Sci. 2012, 3, 3192.
pmid: 23050075 |
| [71] |
Li, B.; Wang, S.-Q.; Liu, B.; Shi, B.-F. Org. Lett. 2015, 17, 1200.
|
| [72] |
Liu, W.; Tan, L.; Zhou, P.; Chen, C.; Zhang, Q. Synthesis 2013, 45, 2600.
|
| [73] |
Su, Y.; Shi, Y.; Chang, B.; Wu, L.; Chong, S.; Zhang, W.; Huang, D.; Wang, K.; Hu, Y. Chin. J. Org. Chem. 2018, 38, 1454 (in Chinese).
|
|
(苏瀛鹏, 石娅, 常兵兵, 吴丽丽, 种思颖, 张为钢, 黄丹凤, 王克虎, 胡雨来, 有机化学, 2018, 38, 1454.)
doi: 10.6023/cjoc201712042 |
|
| [74] |
Rit, R. K.; Yadav, M. R.; Ghosh, K.; Shankar, M.; Sahoo, A. K. Org. Lett. 2014, 16, 5258.
|
| [75] |
Xiong, H. Y.; Cahard, D.; Pannecoucke, X.; Besset, T. Eur. J. Org. Chem. 2016, 2016, 3625.
|
| [76] |
Yang, X.; Sun, Y.; Sun, T. Y.; Rao, Y. Chem. Commun. 2016, 52, 6423.
|
| [1] | 郭怡诗, 桑田, 刘岩, 刘平. 碱催化的β-酮腈与偶氮二甲酸酯的化学选择性取代反应[J]. 有机化学, 2025, 45(4): 1249-1260. |
| [2] | 白磊阳, 付蓓, 刘海平, 淳享, 姜雪峰. 白花前胡素E的全合成研究[J]. 有机化学, 2025, 45(3): 1009-1020. |
| [3] | 杨俊峰, 赵艳秋, 时磊. 电子供体-受体(EDA)复合物驱动的N-α位C—H键活化[J]. 有机化学, 2025, 45(2): 559-573. |
| [4] | 张朝威, 徐兵斌, 刘文龙, 赵敬, 段伟良. 钯催化不对称碳氢键活化合成平面手性二茂铁磺酰胺化合物[J]. 有机化学, 2025, 45(2): 707-716. |
| [5] | 令天鹏, 秦海涛, 刘峰. C(sp3)—H键对映选择性自由基反应的最新进展[J]. 有机化学, 2025, 45(2): 498-515. |
| [6] | 王淼, 黄雅豪, 胡鹏. 氢原子转移介导的烷烃C(sp3)—H选择性官能团化研究进展[J]. 有机化学, 2025, 45(2): 477-497. |
| [7] | 刘泽水, 郭桢桢, 牛俊龙. 过渡金属催化C—H键硅基化反应构建硅杂环研究进展[J]. 有机化学, 2025, 45(2): 423-447. |
| [8] | 袁晨晖, 焦雷. 手性配体在钯催化配位辅助对映选择性C(sp3)—H键官能团化反应中的应用[J]. 有机化学, 2025, 45(2): 602-619. |
| [9] | 王君伟, 薛皓, 曲英瑜, 姜若楠, 闫法超, 刘会. 过渡金属催化联烯胺类化合物的碳氢化反应研究进展[J]. 有机化学, 2025, 45(1): 151-167. |
| [10] | 李平, 张寅, 杨子琪, 郝文娟, 姜波. 利用碱促进环外1,3-二羰化合物的解构反应合成腙化的1,n-二羰化合物及其生物活性检测[J]. 有机化学, 2024, 44(9): 2777-2784. |
| [11] | 李龙龙, 何欣悦, 周龙生, 曲亨通, 冯承涛, 徐坤. 硫氰酸铵促进的[3+3]环化反应合成5-芳基吡唑并[1,5-a]嘧啶[J]. 有机化学, 2024, 44(9): 2832-2840. |
| [12] | 朱洁, 汤思丹, 阚秀妹, 凡士柱, 王鹏飞, 杨培俊. 溶剂控制三氟甲烷磺酸钪催化2-(杂)芳基-N-磺酰基吖丁啶开环反应: 烯丙胺/1,3-噁嗪衍生物的合成[J]. 有机化学, 2024, 44(9): 2796-2809. |
| [13] | 杜佳言, 刘俊涛, 刘桂霞, 黄正. 钴催化末端烯烃区域和立体选择性异构合成反式-2-烯烃[J]. 有机化学, 2024, 44(9): 2889-2897. |
| [14] | 赵明, 颜瑞, 陈虎. 氮杂环卡宾催化醛类化合物的极性反转[J]. 有机化学, 2024, 44(7): 2204-2215. |
| [15] | 曹茜娴, 由君, 刘其业, 刘波, 喻艳超, 武文菊. (4S,4'S)-2,2'-(4,6-二苯并呋喃二基)双[4,5-二氢-4-苯基噁唑]-镍(II)配合物催化高对映选择性氰亚胺的环加成反应[J]. 有机化学, 2024, 44(7): 2315-2332. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||