

硒介导异腈与邻氨基苯酚环化反应合成2-胺基苯并噁唑
收稿日期: 2024-07-01
修回日期: 2024-08-18
网络出版日期: 2024-09-30
基金资助
湖南省自然科学基金(2024JJ7182)
Selenium Mediated Cyclization Reaction of Isonitriles with o-Aminophenols to Synthesize 2-Aminobenzoxazoles
Received date: 2024-07-01
Revised date: 2024-08-18
Online published: 2024-09-30
Supported by
Natural Science Foundation of Hunan Province(2024JJ7182)
王峥 , 兰羽琴 , 周芷怡 , 谭英姿 , 王宗成 . 硒介导异腈与邻氨基苯酚环化反应合成2-胺基苯并噁唑[J]. 有机化学, 2025 , 45(4) : 1379 -1385 . DOI: 10.6023/cjoc202407001
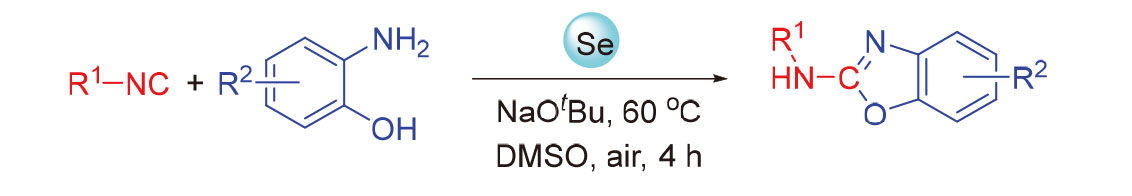
Selenium mediated cyclization reaction of isonitriles with o-aminophenols leading to 2-aminobenzoxazoles has been reported. The present method, which simply utilizes element selenium as promoter and tBuONa as base, provides a green and efficient approach to synthesize various 2-aminobenzoxazoles under mild conditions. This reaction has the advantages of simple operation and metal-free condition. 2-Aminobenzothiazole and 2-aminobenzimidazole could also be synthesized through this methodology.
Key words: metal-free; selenium; isonitriles; o-aminophenols; 2-aminobenzoxazoles
| [1] | (a) Alper-Hayta, S.; Arisoy, M.; Temiz-Arpaci, ?.; Yildiz, I.; Aki, E.; ?zkan, S.; Kaynak, F. Eur. J. Med. Chem. 2008, 43, 2568. |
| [1] | (b) Safak, C.; Erdogan, H.; Palaska, E.; Sunal, R.; Duru, S. J. Med. Chem. 1992, 35, 1296. |
| [1] | (c) Feng, M. H.; Tang, B. Q.; Liang, S. H.; Jiang, X. F. Curr. Top. Med. Chem. 2016, 16, 1200. |
| [1] | (d) Hal, I. H.; Peaty, N. J.; Henry, J. R.; Easmon, J.; Heinisch, G.; Pürstinger, G. Arch. Pharm. 1999, 332, 115. |
| [1] | (e) Hou, J.-C.; Ji, H.-T.; Lu, Y.-H.; Wang, J.-S.; Xu, Y.-D.; Zeng, Y.-Y.; He, W.-M. Chin. Chem. Lett. 2024, 35, 109514. |
| [1] | (f) Chen, X.; Ouyang, W.-T.; Li, X.; He, W.-M. Chin. J. Org. Chem. 2023, 43, 4213 (in Chinese). |
| [1] | (陈祥, 欧阳文韬, 李潇, 何卫民, 有机化学, 2023, 43, 4213.) |
| [2] | (a) Pochetti, G.; Mitro, N.; Lavecchia, A.; Gilardi, F.; Besker, N.; Scotti, E.; Aschi, M.; Re, N.; Fracchiolla, G.; Laghezza, A.; Tortorella, P.; Montanari, R.; Novellino, E.; Mazza, F.; Crestani, M.; Loiodice, F. J. Med. Chem. 2010, 53, 4354. |
| [2] | (b) Kohler, P. C.; Ritschel, T.; Schweizer, W. B.; Klebe, G.; Diederich, F. Chem.-Eur. J. 2009, 15, 10809. |
| [3] | Chai, J.-Y.; Jung, B.-K.; Hong, S.-J. Korean J. Parasitol. 2021, 59, 189. |
| [4] | Liu, K. G.; Lo, J. R.; Comery, T. A.; Zhang, G. M.; Zhang, J. Y.; Kowal, D. M.; Smith, D. L.; Di, L.; Kerns, E. H.; Schechter, L. E.; Robichaud, A. J. Bioorg. Med. Chem. Lett. 2009, 19, 1115. |
| [5] | Muro, F.; Iimura, S.; Yoneda, Y.; Chiba, J.; Watanabe, T.; Setoguchi, M.; Takayama, G.; Yokoyama, M.; Takashi, T.; Nakayama, A.; Machinaga, N. Bioorg. Med. Chem. 2009, 17, 1232. |
| [6] | Kablaoui, N.; Patel, S.; Shao, J.; Demian, D.; Hoffmaster, K.; Berlioz, F.; Vazquez, M. L.; Moore, W. M.; Nugent, R. A. Bioorg. Med. Chem. Lett. 2013, 23, 907. |
| [7] | Seregin, I.; Gevorgyan, V. Chem. Soc. Rev. 2007, 36, 1173. |
| [8] | You, L.; Yuan, J.; Yang, L.; Xiao, Y.; Mao, P. Chin. J. Org. Chem. 2016, 36, 2634 (in Chinese). |
| [8] | (游利琴, 袁金伟, 杨亮茹, 肖咏梅, 毛璞, 有机化学, 2016, 36, 2634.) |
| [9] | (a) Vardhan Reddy, K. H.; Anil Kumar, B. S. P.; Prakash Reddy, V.; Uday Kumar, R.; Nageswar, Y. V. D. RSC Adv. 2014, 4, 45579. |
| [9] | (b) Yamato, M.; Takeuchi, Y.; Hattori, K.; Hashigaki, K. Chem. Pharm. Bull. 1984, 32, 3053. |
| [9] | (c) Saladino, R.; Crestini, C.; Occhionero, F.; Nicoletti, R. Synth. Commun. 1996, 26, 3241. |
| [9] | (d) K?vér, J.; Tímár, T.; Tompa, J. Synthesis 1994, 1124. |
| [9] | (e) Gu, J.; Cai, C. Synlett 2015, 26, 639. |
| [10] | (a) Wu, Y. Q.; Limburg, D. C.; Wilkinson, D. E.; Hamilton, G. S. J. Heterocycl. Chem. 2003, 40, 191. |
| [10] | (b) El-Faham, A.; Chebbo, M.; Abdul-Ghani, M.; Younes, G. Heterocycl. Chem. 2006, 43, 599. |
| [10] | (c) Cioffi, C. L.; Lansing, J. J.; Yüksel, H. J. Org. Chem. 2010, 75, 7942. |
| [10] | (d) Zhang, X. Y.; Jia, X. F.; Wang, J. J.; Fan, X. S. Green Chem. 2011, 13, 413. |
| [10] | (e) Kasthuri, M.; Sharath Babu, H.; Shiva Kumar, C.; Nagendra Kumar, P. V. Synlett 2015, 26, 897. |
| [10] | (f) Lin, C. C.; Hsieh, T. H.; Liao, P. Y.; Liao, Z. Y.; Chang, C. W.; Shih, Y. C.; Yeh, W. H.; Chien, T. C. Org. Lett. 2014, 16, 892. |
| [11] | (a) Kim, J. Y.; Cho, S. H.; Joseph, J.; Chang, S. Angew. Chem.,Int. Ed. 2010, 49, 9899. |
| [11] | (b) Li, Y. M.; Liu, J.; Xie, Y. S.; Zhang, R.; Jin, K.; Wang, X. N.; Duan, C. Y. Org. Biomol. Chem. 2012, 10, 3715. |
| [11] | (c) Li, Y. M.; Xie, Y. S.; Zhang, R.; Jin, K.; Wang, X. N.; Duan, C. Y. J. Org. Chem. 2011, 76, 5444. |
| [11] | (d) Monguchi, D.; Fujiwara, T.; Furukawa, H.; Mori, A. Org. Lett. 2009, 11, 1607. |
| [11] | (e) Pal, P.; Giri, A. K.; Singh, H.; Ghosh, S. C.; Panda, A. B. Chem. Asian J. 2014, 9, 2392. |
| [11] | (f) Xie, Y. J.; Qian, B.; Xie, P.; Huang, H. M. Adv. Synth. Catal. 2013, 355, 1315. |
| [11] | (g) Gao, W. J.; Li, W. C.; Zeng, C. C.; Tian, H. Y.; Hu, L. M.; Little, R. D. J. Org. Chem. 2014, 79, 9613. |
| [12] | (a) Gilchrist, T. L.; John Harris, C.; King, F. D.; Peek, M. E. J. Chem. Soc., 1988, 2169. |
| [12] | (b) Morofuji, T.; Shimizu, A.; Yoshida, J. Chem.-Eur. J. 2015, 21, 3211. |
| [13] | (a) Wang, H.; Xu, B. Chin. J. Org. Chem. 2015, 35, 588 (in Chinese). |
| [13] | (王浩, 许斌, 有机化学, 2015, 35, 588.) |
| [13] | (b) Lv, Y.; Bao, P.; Yue, H.; Li, J-S.; Wei, W. Green Chem. 2019, 21, 6051. |
| [13] | (c) Ma, C.; Li, X.; Chen, X.; He, X.; Zhang, S-T.; Jiang, Y-Q.; Yu, B. Org. Lett., 2023, 25, 8016. |
| [14] | (a) Zhu, T.-H.; Wang, S.-Y.; Wang, G.-N.; Ji, S.-J. Chem.-Eur. J. 2013, 19, 5850. |
| [14] | (b) Zhu, T.-H.; Wang, S.-Y.; Wang, G.-N.; Ji, S.-J. Adv. Synth. Catal. 2014, 356, 509. |
| [15] | Liu, J.; Hoover, J. M. Org. Lett. 2019, 21, 4510. |
| [16] | Liu, B.; Yin, M.; Gao, H.; Wu, W.; Jiang, H. J. Org. Chem. 2013, 78, 3009. |
| [17] | Vlaar, T.; Cioc, R. C.; Mampuys, P.; Maes, B. U. W.; Orru, R. V. A.; Ruijter, E. Angew. Chem., Int. Ed. 2012, 51, 13058. |
| [18] | Wang, G.-N.; Zhu, T.-H.; Wang, S.-Y.; Wei, T.-Q.; Ji, S.-J. Tetrahedron 2014, 70, 8079. |
| [19] | (a) Ji, H.-T.; Jiang, J.; He, W.-B.; Lu, Y.-H.; Liu, Y.-Y.; Li, X.; He, W-M. J. Org. Chem. 2024, 89, 4113. |
| [19] | (b) Ji, H.-T.; Luo, Q.-X.; Wang, K.-L.; Ouyang, W.-T.; Li, H.-X.; He, W.-M. Green Chem. 2023, 25, 7983. |
| [19] | (c) Kihlberg, T.; Karimi, F.; L?ngstr?m, B. J. Org. Chem. 2002, 67, 3687. |
| [20] | (a) Fei, N.; Wang, Y.; Gu, Y.; Wang, Z.; Zhu, Y.; Li, Y. J. Org. Chem. 2023, 88, 13042. |
| [20] | (b) Lin, J.-X.; Liu, G.-H.; Liu, L.-Q.; Wang, Y.-C.; He, Y. J. Org. Chem. 2024, 89, 101. |
| [20] | (c) Zhong, W.; Li, M.; Jin, Y.; Jiang, H.; Wu, W. Chem. Commun. 2022, 58, 6522. |
| [20] | (d) Shao, L.; Li, Y.; Lu, J.; Jiang, X. Org. Chem. Front. 2019, 6, 2999. |
| [21] | (a) Duangkamol, C.; Phakhodeea, W.; Pattarawarapan, M. Synthesis 2020, 52, 1981. |
| [21] | (b) Tran, D. T.; Huynh, T. N.; Nguyen, P. C.; Phan, N. T. S.; Nguyen, T. T. Tetrahedron Lett. 2023, 122, 154510. |
| [21] | (c) Xu, Y.; Li, F.; Zhao, N.; Su, J.; Wang, C.; Wang, C.; Li, Z.; Wang, L. Green Chem. 2021, 23, 8047. |
/
| 〈 |
|
〉 |