

2-取代的3H-吲哚-3-酮类化合物参与的C2位手性吲哚啉-3-酮类化合物的不对称合成研究进展
收稿日期: 2024-10-16
修回日期: 2026-11-26
网络出版日期: 2024-12-06
基金资助
云南省基础研究计划(202401AT070041); 云南省教育厅科学研究基金(2024J0564); 云南省教育厅科学研究基金(2024Y456)
Research Progress in Asymmetric Synthesis of C2 Chiral Indolin-3-ones Involving 2-Substituted-3H-indol-3-ones
Received date: 2024-10-16
Revised date: 2026-11-26
Online published: 2024-12-06
Supported by
Yunnan Fundamental Research Projects(202401AT070041); Scientific Research Fund of Education Department of Yunnan Province(2024J0564); Scientific Research Fund of Education Department of Yunnan Province(2024Y456)
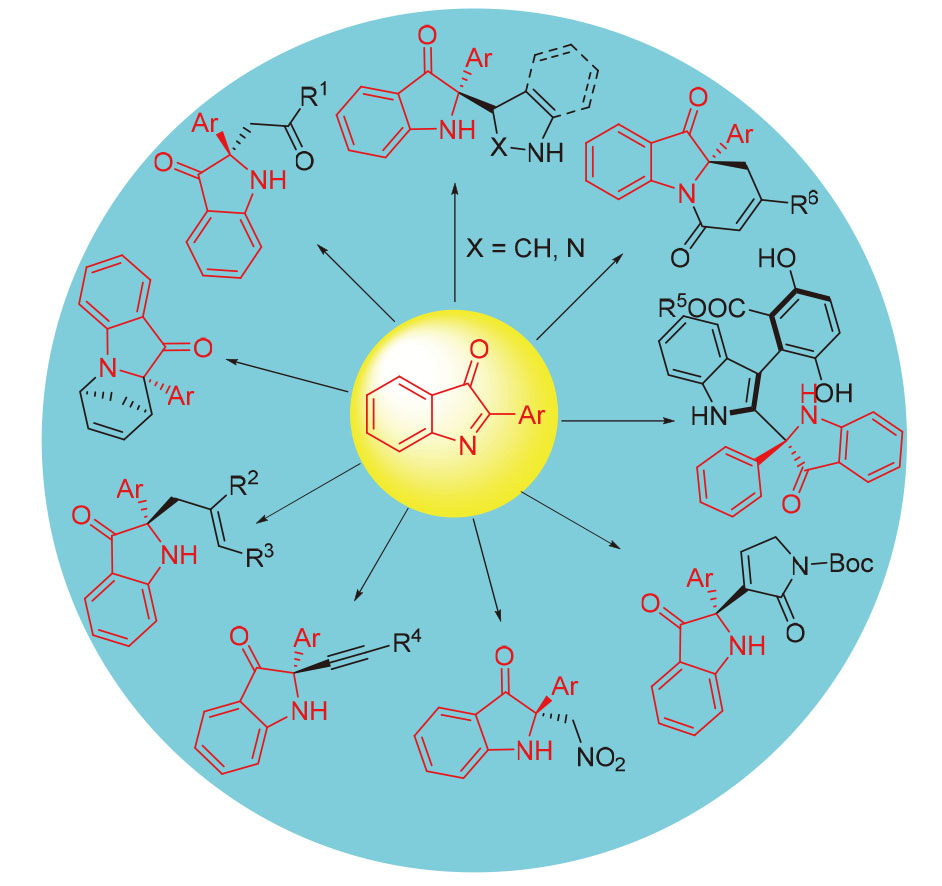
含有2,2-二取代吲哚啉-3-酮类骨架的化合物在天然产物、手性药物等诸多领域有着广泛应用. 通过不对称催化高效构建2,2-二取代吲哚啉-3--酮类化合物受到越来越广泛的关注. 综述了近些年国内外在以2-取代的3H-吲哚-3-酮类化合物为底物的不对称催化合成2,2-二取代吲哚啉-3-酮类化合物方面取得的研究进展, 主要从不对称aza- Friedel-Crafts反应、Mannich反应、aza-Diels-Alder反应、aza-Henry反应、aza-Morita-Baylis-Hillman反应以及其他反应等方面进行讨论, 为今后不对称合成C2季碳中心吲哚啉-3-酮类化合物研究提供参考.
关键词: 2-芳基-3H-吲哚-3-酮; 不对称催化; C2季碳中心吲哚啉-3-酮
乔秀秀 , 李倩 , 赵世娜 , 魏瑞琪 , 马桃 , 何永辉 , 赵晓静 . 2-取代的3H-吲哚-3-酮类化合物参与的C2位手性吲哚啉-3-酮类化合物的不对称合成研究进展[J]. 有机化学, 2025 , 45(4) : 1166 -1177 . DOI: 10.6023/cjoc202407039
Compounds containing 2,2-disubstituted indoline-3-one skeletons are widely used in many fields such as natural products and chiral drugs. The efficient construction of 2,2-disubstituted indolin-3-ones by asymmetric catalysis has attracted more and more attention. The research progress in the asymmetric synthesis of 2,2-disubstituted indolin-3-ones using 2-sub- stituted indolin-3-ones as substrates in recent years is reviewed, including asymmetric aza-Friedel-Crafts reaction, Mannich reaction, aza-Diels-Alder reaction, aza-Henry reaction, aza-Morita-Baylis-Hillman reaction and other reactions. It provides a reference for the future asymmetric synthesis of C2 quaternary carbon center indolin-3-one compounds.

| [1] | Dhote, P. S.; Patel, P.; Vanka, K.; Ramana, C. V. Org. Biomol. Chem. 2021, 19, 7970. |
| [2] | Liu, J.-F.; Jiang, Z.-Y.; Wang, R.-R.; Zheng, Y.-T.; Chen, J.-J.; Zhang, X.-M.; Ma, Y.-B.; Isatisine, A. Org. Lett. 2007, 9, 4127. |
| [3] | Váradi, A.; Marrone, G. F.; Palmer, T. C.; Narayan, A.; Szabó, M. R.; Le Rouzic, V.; Grinnell, S. G.; Subrath, J. J.; Warner, E.; Kalra, S.; Hunkele, A.; Pagirsky, J.; Eans, S. O.; Medina, J. M.; Xu, J.; Pan, Y.-X.; Borics, A.; Pasternak, G. W.; McLaughlin, J. P.; Majumdar, S. J. Med. Chem. 2016, 59, 8381. |
| [4] | (a) Wang, S.-G.; You, S.-L. Angew. Chem.,Int. Ed. 2014, 53, 2194. |
| [4] | (b) You, S.-L.; Cai, Q.; Zeng, M. Chem. Soc. Rev. 2009, 38, 2190. |
| [4] | (c) Zhou, D.; Huang, Z.; Yu, X.; Wang, Y.; Li, J.; Wang, W.; Xie, H. Org. Lett. 2015, 17, 5554. |
| [4] | (d) Ling, J.; Lam, S. K.; Lo, B.; Lam, S.; Wong, W.-T.; Sun, J.; Chen, G.; Chiu, P. Org. Chem. Front. 2016, 3, 457. |
| [4] | (e) Svestka, D.; Otevrel, J.; Bobal, P. Adv. Synth. Catal. 2022, 364, 2174. |
| [5] | Yin, Q.; You, S.-L. Chem. Sci. 2011, 2, 1344. |
| [6] | Rueping, M.; Raja, S.; Nú?ez, A. Adv. Synth. Catal. 2011, 353, 563. |
| [7] | Nakamura, S.; Matsuda, N.; Ohara, M. Chem.-Eur. J. 2016, 22, 9478. |
| [8] | (a) Yarlagadda, S.; Sridhar, B.; Subba Reddy, B. V. Chem. Asian J. 2018, 13, 1327. |
| [8] | (b) Dong, C.-L.; Ding, X.; Huang, L.-Q.; He, Y.-H.; Guan, Z. Org. Lett. 2020, 22, 1076. |
| [8] | (c) Lu, F.-Y.; Chen, Y.-J.; Chen, Y.; Ding, X.; Guan, Z.; He, Y.-H. Chem. Commun. 2020, 56, 623. |
| [9] | Ma, T.; He, Y.; Qiao, X.-X.; Zou, C.-P.; Wu, X.-X.; Li, G.; Zhao, X.-J. Org. Biomol. Chem. 2023, 21, 489. |
| [10] | Qiao, X.-X.; He, Y.; Ma, T.; Zou, C.-P.; Wu, X.-X.; Li, G.; Zhao, X.-J. Chem. Eur. J. 2023, 29, e202203914. |
| [11] | Zheng, J.; Rong, M.-Y.; Feng, F.-F.; Zhang, F.-G.; Cheung, C. W.; Ma, J.-A. Asian J. Org. Chem. 2023, 12, e202300204. |
| [12] | Merad, J.; Lalli, C.; Bernadat, G.; Maury, J.; Masson, G. Chem. Eur. J. 2018, 24, 3925. |
| [13] | (a) Reddy, K. N.; Rao, M. V. K.; Sridhar, B.; Subba Reddy, B. V. Chem. Asian J. 2019, 14, 2958. |
| [13] | (b) Wu, H.-C.; Wang, C.; Chen, Y.-H.; Liu, Y.-K. Chem. Commun. 2021, 57, 1762. |
| [13] | (c) Parmar, D.; Sugiono, E.; Raja, S.; Rueping, M. Chem. Rev. 2014, 114, 9047. |
| [13] | (d) Zhu, Y.; Li, Y.; Meng, Q.; Li, X. Org. Chem. Front. 2016, 3, 709. |
| [13] | (e) Ting, A.; Schaus, S. E. Eur. J. Org. Chem. 2007, 2007, 5797. |
| [13] | (f) Córdova, A. Acc. Chem. Res. 2004, 37, 102. |
| [13] | (g) List, B. J. Am. Chem. Soc. 2000, 122, 9336. |
| [14] | Li, L.; Han, M.; Xiao, M.; Xie, Z. Synlett 2011, 2011, 1727. |
| [15] | Rueping, M.; Rasappan, R.; Raja, S. Helv. Chim. Acta 2012, 95, 2296. |
| [16] | Li, J.-S.; Liu, Y.-J.; Zhang, G.-W.; Ma, J.-A. Org. Lett., 2017, 19, 6364. |
| [17] | Li, J.-S.; Liu, Y.-J.; Li, S.; Ma, J.-A. Chem. Commun. 2018, 54, 9151. |
| [18] | An, J.-X.; Yang, F.-F.; Wang, P.; Gu, Z.-C.; Li, Y.; Chen, L.; Zhao, Y.-L.; He, B. RSC Adv. 2022, 12, 7040. |
| [19] | (a) Masson, G.; Lalli, C.; Benohoud, M.; Dagousset, G. Chem. Soc. Rev. 2013, 42, 902. |
| [19] | (b) Eschenbrenner-Lux, V.; Kumar, K.; Waldmann, H. Angew. Chem., Int. Ed. 2014, 53, 11146. |
| [19] | (c) Cao, M.-H.; Green, N. J.; Xu, S.-Z. Org. Biomol. Chem. 2017, 15, 3105. |
| [19] | (d) Hatanaka, Y.; Nantaku, S.; Nishimura, Y.; Otsuka, T.; Sekikaw, T. Chem. Commun. 2017, 53, 8996. |
| [19] | (e) Wang, C.; Li, Y.; Wu, Y.; Wang, Q.; Shi, W.; Yuan, C.; Zhou, L.; Xiao, Y.; Guo, H. Org. Lett. 2018, 20, 2880. |
| [19] | (f) Tomifuji, R.; Kurahashi, T.; Matsubara, S. Chem.-Eur. J. 2019, 25, 8987. |
| [19] | (g) Weilbeer, C.; Sickert, M.; Naumov, S.; Schneider, C. Chem.-Eur. J. 2017, 23, 513. |
| [20] | Rueping, M.; Raja, S. Beilstein J. Org. Chem. 2012, 8, 1819. |
| [21] | Liu, J.-X.; Zhou, Q.-Q.; Deng, J.-G.; Chen, Y.-C. Org. Biomol. Chem. 2013, 11, 8175. |
| [22] | Yadav, J.; Dolas, A. J.; Iype, E.; Rangan, K.; Ohshita, J.; Kumar, D.; Kumar, I. J. Org. Chem. 2021, 86, 17213. |
| [23] | Zhao, Q.; Li, Y.; Zhang, Q.-X.; Cheng, J.-P.; Li, X. Angew. Chem., Int. Ed. 2021, 60, 17608. |
| [24] | (a) Liu, X.; Zheng, K.; Feng, X. Synthesis 2014, 46, 2241. |
| [24] | (b) Hack, D.; Blümel, M.; Chauhan, P.; Philipps, A. R.; Enders, D. Chem. Soc. Rev. 2015, 44, 6059. |
| [24] | (c) Saha, P.; Saikia, A. K. Org. Biomol. Chem. 2018, 16, 2820. |
| [24] | (d) Bakhtiari, A.; Safaei-Ghomi, J. Synlett 2019, 30, 1738. |
| [25] | Zhang, Q.-X.; Li, Y.; Wang, J.; Yang, C.; Liu, C.-J.; Li, X.; Cheng, J.-P. Angew. Chem., Int. Ed. 2020, 59, 4550. |
| [26] | (a) Blay, G.; Brines, A.; Monleón, A.; Pedro, J. R. Chem.-Eur. J. 2012, 18, 2440. |
| [26] | (b) Zhang, F.-G.; Ma, H.; Nie, J.; Zheng, Y.; Gao, Q.; Ma, J.-A. Adv. Synth. Catal. 2012, 354, 1422. |
| [26] | (c) Liu, T.-L.; Zhang, H.-X.; Zheng, Y.; Yao, Q.; Ma, J.-A. Chem. Commun. 2012, 48, 12234. |
| [26] | (d) De Munck, L.; Monleón, A.; Vila, C.; Pedro, J. R. Adv. Synth. Catal. 2017, 359, 1582. |
| [27] | Wu, X.-X.; Ma, T.; Qiao, X.-X.; Zou, C.-P.; Li, G.; He, Y.; Zhao, X.-J. Chem. Asian. J. 2023, 18, e202300526. |
| [28] | (a) Fang, X.; Wang, C.-J. Chem. Commun. 2015, 51, 1185. |
| [28] | (b) Otocka, S.; Kwiatkowska, M.; Madalińska, L.; Kie?basiński, P. Chem. Rev. 2017, 117, 4147. |
| [28] | (c) Ping, X.-N.; Wei, P.-S.; Zhu, X.-Q.; Xie, J.-W. J. Org. Chem. 2017, 82, 2205. |
| [28] | (d) Devannah, V.; Sharma, R.; Watson, D. A. J. Am. Chem. Soc. 2019, 141, 8436. |
| [28] | (e) B?r, A.; B?r, S. I.; Schobert, R. Org. Biomol. Chem. 2020, 18, 7565. |
| [29] | Parra, A.; Alfaro, R.; Marzo, L.; Moreno-Carrasco, A.; García Ruano, J. L.; Alemán, J. Chem. Commun. 2012, 48, 9759. |
| [30] | Wei, Y.; Shi, M. Chem. Rev. 2013, 113, 6659. |
| [31] | Wu, X.-X.; He, Y.; Qiao, X.-X.; Ma, T.; Zou, C.-P.; Li, G.; Zhao, X.-J. J. Org. Chem. 2023, 88, 6599. |
| [32] | (a) Cheng, J. K.; Xiang, S.-H.; Li, S.; Ye, L.; Tan, B. Chem. Rev. 2021, 121, 4805. |
| [32] | (b) Da, B.-C.; Xiang, S.-H.; Li, S.; Tan, B. Chin. J. Chem. 2021, 39, 1787. |
| [32] | (c) Wang, Y. B.; Tan, B. Acc. Chem. Res. 2018, 51, 534. |
| [32] | (d) Kitagawa, O. Acc. Chem. Res. 2021, 54, 719. |
| [33] | Yuan, X.; Wu, X.; Peng, F.; Yang, H.; Zhu, C.; Fu, H. Chem. Commun. 2020, 56, 12648. |
| [34] | Fang, S.; Jin, S.; Ma, R.; Lu, T.; Du, D. Org. Lett. 2019, 21, 5211. |
| [35] | Yuan, X.; Wu, X.; Zhang, P.; Peng, F.; Liu, C.; Yang, H.; Zhu, C.; Fu, H. Org. Lett. 2019, 21, 2498. |
/
| 〈 |
|
〉 |