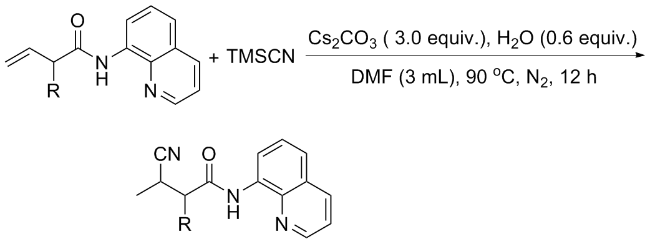
向100 mL的单口烧瓶中加入二异丙基氨基锂(LDA) (11.8 mL, 23.5 mmol), 降温至0 ℃, 将3-丁烯酸 (1 mL, 11.77 mmol)和10 mL的四氢呋喃溶液缓慢加入, 反应45 min, 加入1.1 equiv.的卤代烷烃试剂, 继续反应30 min, 室温下再搅拌1 h. 反应结束后用10%的稀盐酸调整pH=2.5. 用二氯甲烷(15 mL×3)萃取, 合并有机相后, 用饱和食盐水(25 mL×2)洗涤, 最后用无水硫酸钠干燥, 减压浓缩除去乙酸乙酯得到粗产物.
向250 mL的单口烧瓶中加入二氯甲烷(30 mL), 丁烯酸或上述方法制备的取代的丁烯酸(12 mL), 8-氨基喹啉(1.44 g, 10 mL)和N,N,N',N'-四甲基-O-(7-氮杂苯并三唑-1-基)六氟磷酸脲(HATU) (4.94 g, 13 mmol), 室温下搅拌3~5 min,然后加入吡啶(2.6 mL, 20 mmol), 继续反应16 h. 反应结束后向体系中加入200 mL的二氯甲烷, 分别用100 mL的饱和碳酸氢钠溶液和100 mL的饱和食盐水各洗涤一次, 有机相用无水硫酸钠干燥,减压浓缩, 通过柱层析[V(石油醚)∶V(乙酸乙酯)=20∶1]纯化得到原料1.
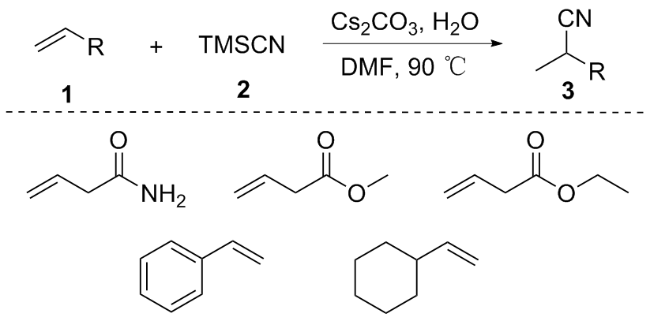
N-(喹啉-8-基)丁-3-烯酰胺(1a): 黄色油状液体. 1H NMR (400 MHz, CDCl3) δ: 9.86 (s, 1H), 8.77 (dd, J=7.6, 1.4 Hz, 1H), 8.63 (dd, J=4.2, 1.7 Hz, 1H), 7.89 (dd, J=8.3, 1.7 Hz, 1H), 7.38~7.26 (m, 2H), 7.21 (dd, J=8.3, 4.2 Hz, 1H), 6.13 (dd, J=16.9, 10.3 Hz, 1H), 5.40~5.23 (m, 2H), 3.29 (dt, J=7.1, 1.4 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ: 168.75, 147.89, 138.01, 135.84, 134.17, 131.02, 127.52, 126.85, 121.31, 121.28, 119.52, 116.03, 42.80; HRMS (ESI-TOF) calcd for C13H13N2O [M+H]+ 213.1028, found 213.1029.
2-(环丙基甲基)-N-(喹啉-8-基)丁-3-烯酰胺(1b): 黄色油状液体. 1H NMR (400 MHz, CDCl3) δ: 9.87 (s, 1H), 8.69~8.63 (m, 2H), 8.01 (dd, J=8.3, 1.7 Hz, 1H), 7.42~7.34 (m, 2H), 7.31 (dd, J=8.3, 4.2 Hz, 1H), 5.96 (ddd, J=17.1, 10.2, 8.6 Hz, 1H), 5.27~5.13 (m, 2H), 3.19 (d, J=8.0 Hz, 1H), 1.75~1.65 (m, 1H), 1.58~1.52 (m, 1H), 0.68 (dd, J=7.8, 6.6 Hz, 1H), 0.40~0.23 (m, 2H), 0.01 (d, J=4.5 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ: 172.15, 148.23, 138.57, 137.09, 136.31, 134.55, 127.95, 127.42, 121.56, 121.46, 117.77, 116.41, 53.95, 37.29, 9.04, 4.72, 4.60; HRMS (ESI-TOF) calcd for C17H19N2O [M+H]+ 267.1501, found 267.1499.
N-(喹啉-8-基)-2-乙烯基戊-4-烯酰胺(1c): 黄色油状液体. 1H NMR (400 MHz, CDCl3) δ: 10.00 (s, 1H), 8.88~8.73 (m, 2H), 8.13 (dd, J=8.3, 1.7 Hz, 1H), 7.57~7.37 (m, 3H), 6.04 (ddd, J=17.2, 10.2, 8.5 Hz, 1H), 5.86 (ddt, J=17.1, 10.2, 6.9 Hz, 1H), 5.51~5.26 (m, 2H), 5.26~5.01 (m, 2H), 3.29 (dt, J=7.5, 0.9 Hz, 1H), 2.83~2.70 (m, 1H), 2.50 (dd, J=14.3, 7.2 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ: 171.30, 148.25, 138.57, 136.38, 136.30, 135.39, 134.44, 128.47, 128.37, 127.94, 127.38, 121.58, 118.49, 117.08, 116.45, 53.03, 36.14; HRMS (ESI- TOF) calcd for C16H17N2O [M+H]+ 253.1301, found 253.1302.
2-甲基-N-(喹啉-8-基)丁-3-烯酰胺(1d): 淡黄色油状液体. 1H NMR (400 MHz, CDCl3) δ: 9.96 (s, 1H), 8.72 (ddd, J=9.1, 5.7, 1.7 Hz, 2H), 8.08 (dd, J=8.2, 1.7 Hz, 1H), 7.56~7.33 (m, 3H), 6.04 (ddd, J=17.2, 10.2, 7.8 Hz, 1H), 5.35~5.21 (m, 2H), 3.39~3.24 (m, 1H), 1.38 (d, J=7.0 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 148.25, 138.62, 138.00, 136.31, 127.95, 127.41, 121.56, 121.49, 117.29, 116.38, 47.02, 16.96; HRMS (ESI-TOF) calcd for C14H15N2O [M+H]+ 227.1103, found 227.1102.
2-乙基-N-(喹啉-8-基)丁-3-烯酰胺(1e): 黄色油状液体. 1H NMR (400 MHz, CDCl3) δ: 9.97 (s, 1H), 8.86~8.72 (m, 2H), 8.13 (dd, J=8.3, 1.7 Hz, 1H), 7.63~7.39 (m, 3H), 6.02 (ddd, J=17.2, 10.1, 8.6 Hz, 1H), 5.42~5.20 (m, 2H), 3.18~2.98 (m, 1H), 2.04 (ddd, J=13.7, 7.4, 6.5 Hz, 1H), 1.84~1.64 (m, 1H), 1.01 (t, J=7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 172.14, 148.22, 138.55, 136.83, 136.31, 134.53, 127.94, 127.39, 121.56, 121.48, 118.16, 116.41, 55.12, 25.11, 11.82; HRMS (ESI-TOF) calcd for C15H17N2O [M+H]+ 241.1303, found 241.1305.
N-(喹啉-8-基)-2-乙烯基庚-6-烯酰胺(1f): 淡黄色油状液体. 1H NMR (400 MHz, CDCl3) δ: 9.98 (s, 1H), 8.92~8.70 (m, 2H), 8.13 (dd, J=8.3, 1.7 Hz, 1H), 7.63~7.33 (m, 3H), 6.10~5.98 (m, 1H), 5.93~5.74 (m, 1H), 5.49~5.24 (m, 2H), 5.17~4.92 (m, 2H), 3.35~3.16 (m, 1H), 2.16~1.96 (m, 3H), 1.71 (dddd, J=13.3, 10.6, 7.8, 5.4 Hz, 1H), 1.60~1.43 (m, 2H); 13C NMR (101 MHz, CDCl3) δ: 172.04, 148.23, 138.54, 138.47, 136.96, 136.31, 134.50, 127.94, 127.39, 121.58, 121.51, 118.12, 116.42, 114.78, 53.37, 33.62, 31.39, 26.56; HRMS (ESI-TOF) calcd for C18H21N2O [M+H]+ 281.1603, found 281.1600.
2-环己基-N-(喹啉-8-基)丁-3-烯酰胺(1g): 黄色油状液体. 1H NMR (400 MHz, CDCl3) δ: 9.90 (s, 1H), 8.91~8.74 (m, 2H), 8.09 (dd, J=8.3, 1.7 Hz, 1H), 7.60~7.37 (m, 3H), 6.09~5.87 (m, 1H), 5.45~5.13 (m, 2H), 2.88 (t, J=8.9 Hz, 1H), 1.90~1.59 (m, 5H), 1.36~0.90 (m, 5H); 13C NMR (101 MHz, CDCl3) δ: 172.06, 148.19, 138.47, 136.29, 135.96, 134.48, 127.92, 127.36, 121.56, 121.48, 118.41, 116.42, 60.73, 39.74, 31.47, 30.25, 26.37, 26.22; HRMS (ESI-TOF) calcd for C19H23N2O [M+H]+ 295.1704, found 295.1701.
N-(喹啉-8-基)-2-乙烯基壬酰胺(1h): 白色固体, m.p. 107~108 ℃; 1H NMR (400 MHz, CDCl3) δ: 9.98 (s, 1H), 8.91~8.75 (m, 2H), 8.10 (dd, J=8.3, 1.7 Hz, 1H), 7.63~7.34 (m, 3H), 6.02 (ddd, J=17.1, 10.2, 8.6 Hz, 1H), 5.51~5.20 (m, 2H), 3.17 (q, J=7.7 Hz, 1H), 1.98 (ddd, J=13.1, 8.5, 4.8 Hz, 1H), 1.76~1.60 (m, 1H), 1.49~1.17 (m, 10H), 0.93~0.79 (m, 3H); 13C NMR (101 MHz, CDCl3) δ: 172.20, 148.19, 138.53, 137.15, 136.27, 134.54, 127.91, 127.36, 121.54, 121.45, 117.89, 116.39, 53.54, 31.98, 31.83, 29.49, 29.17, 27.27, 22.65, 14.10; HRMS (ESI-TOF) calcd for C20H27N2O [M+H]+ 311.1994, found 311.2000.
2-(4-甲基苄基)-N-(喹啉-8-基)丁-3-烯酰胺(1i): 黄色油状液体. 1H NMR (400 MHz, CDCl3) δ: 9.91 (s, 1H), 8.87~8.49 (m, 2H), 7.89 (dd, J=8.3, 1.7 Hz, 1H), 7.40 (t, J=8.0 Hz, 1H), 7.30 (dd, J=8.3, 1.4 Hz, 1H), 7.20 (dd, J=8.3, 4.2 Hz, 1H), 7.12 (d, J=8.0 Hz, 2H), 7.00 (d, J=7.9 Hz, 2H), 6.04 (ddd, J=17.1, 10.2, 8.5 Hz, 1H), 5.30~5.15 (m, 2H), 3.57~3.26 (m, 2H), 2.93 (dd, J=13.7, 7.4 Hz, 1H), 2.19 (s, 3H); 13C NMR (101 MHz, CDCl3) δ: 171.29, 148.17, 138.45, 136.59, 136.18, 136.12, 135.71, 134.52, 129.19, 129.16, 127.88, 127.28, 121.62, 121.55, 118.60, 116.45, 55.26, 37.87, 21.12; HRMS (ESI-TOF) calcd for C21H21N2O [M+H]+ 317.1604, found 317.1601.
2-苯乙基-N-(喹啉-8-基)丁-3-烯酰胺(1j): 黄色油状液体. 1H NMR (400 MHz, CDCl3) δ: 9.98 (s, 1H), 8.86~8.68 (m, 2H), 8.13 (dd, J=8.3, 1.7 Hz, 1H), 7.56~7.40 (m, 3H), 7.31~7.16 (m, 5H), 6.05 (ddd, J=17.1, 10.2, 8.6 Hz, 1H), 5.47~5.26 (m, 2H), 3.20 (q, J=7.6 Hz, 1H), 2.73 (dt, J=8.8, 6.2 Hz, 2H), 2.51~2.30 (m, 1H), 2.13~1.89 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 171.76, 148.26, 141.57, 138.58, 136.82, 136.33, 134.51, 128.61, 128.45, 127.40, 125.98, 121.61, 121.59, 118.54, 116.49, 52.60, 33.34, 33.29; HRMS (ESI-TOF) calcd for C21H21N2O [M+H]+ 317.1607, found 317.1605.
2-(4-氟苄基)-N-(喹啉-8-基)丁-3-烯酰胺(1k): 黄色油状液体. 1H NMR (400 MHz, CDCl3) δ: 9.90 (s, 1H), 8.85~8.68 (m, 2H), 8.11 (dd, J=8.3, 1.7 Hz, 1H), 7.54~7.45 (m, 2H), 7.40 (dd, J=8.3, 4.2 Hz, 1H), 7.23~7.17 (m, 2H), 6.96~6.87 (m, 2H), 6.03 (ddd, J=17.3, 10.0, 8.6 Hz, 1H), 5.38~5.21 (m, 2H), 3.48~3.30 (m, 2H), 3.00~2.89 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 171.03, 162.76, 160.33, 148.26, 148.23, 138.47, 136.33, 136.30, 136.13, 134.82, 134.79, 134.31, 130.71, 130.63, 127.91, 127.37, 127.33, 121.68, 121.64, 121.60, 118.85, 116.46, 115.24, 115.03, 55.25, 37.32; HRMS (ESI-TOF) calcd for C20H18FN2O [M+H]+ 321.3710, found 321.3709.
2-(环丁基甲基)-N-(喹啉-8-基)丁-3-烯酰胺(1l): 黄色油状液体. 1H NMR (400 MHz, CDCl3) δ: 9.94 (s, 1H), 8.84~8.72 (m, 2H), 8.15 (dd, J=8.3, 1.7 Hz, 1H), 7.63~7.34 (m, 3H), 6.01 (ddd, J=17.1, 10.1, 8.7 Hz, 1H), 5.38~5.18 (m, 2H), 3.20~2.99 (m, 1H), 2.50~2.34 (m, 1H), 2.16~1.99 (m, 3H), 1.89~1.64 (m, 5H); 13C NMR (101 MHz, CDCl3) δ: 170.34, 148.37, 138.40, 136.41, 133.87, 127.93, 127.38, 122.05, 121.74, 121.39, 116.89, 49.27, 37.41, 33.86, 28.44, 28.23, 28.14, 18.34, 15.48; HRMS (ESI-TOF) calcd for C18H21N2O [M+H]+ 281.1604, found 281.1606.
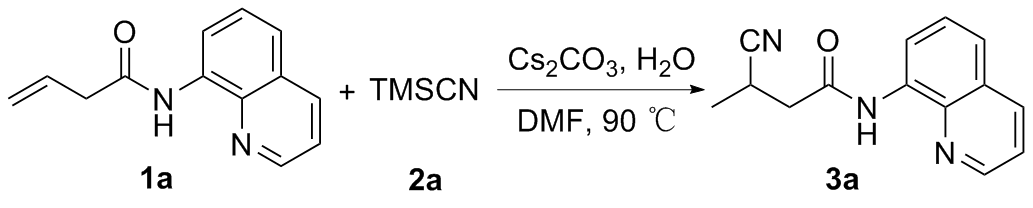
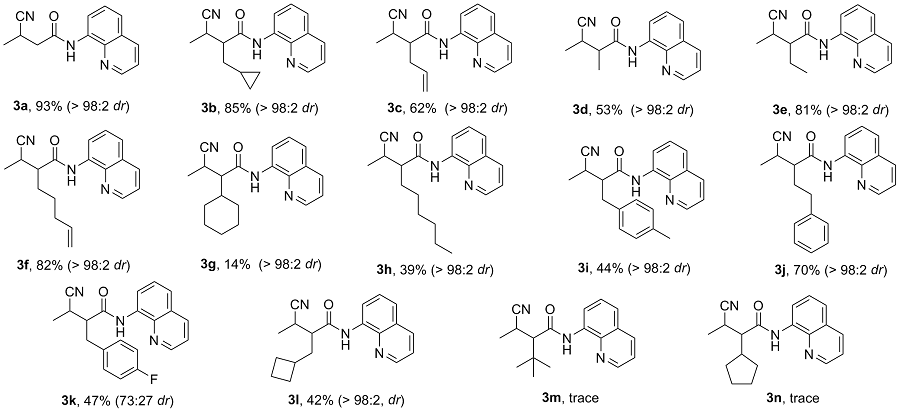
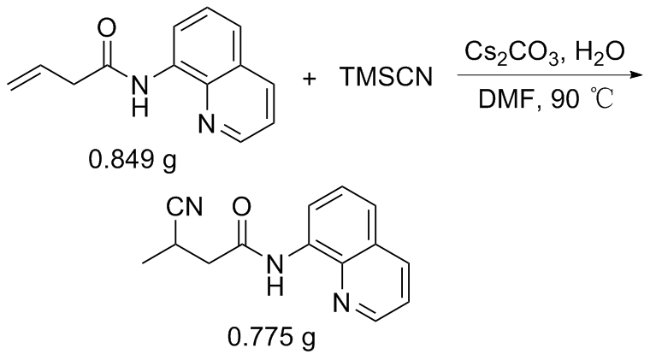
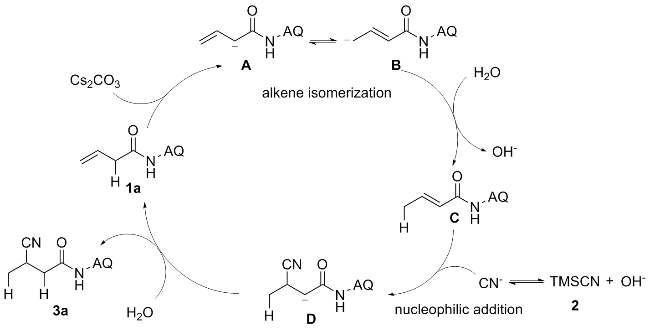
向10 mL反应管中加入1a (42.6 mg, 0.2 mmol)、碳酸铯(70.1 mg, 0.2 mmol)、TMSCN (0.4 mmol)、去离子水(0.12 mmol)和2 mL的DMF, 氮气保护, 在90 ℃下反应12 h. 反应结束后使用乙酸乙酯(30 mL)萃取两次, 合并有机相, 用无水硫酸钠干燥, 减压浓缩, 通过柱层析[V(石油醚)∶V(乙酸乙酯)=20∶1]纯化得到3-氰基- N-(喹啉-8-基)丁酰胺(3a), 黄色油状液体. 1H NMR (400 MHz, CDCl3) δ: 9.81 (s, 1H), 8.73~8.63 (m, 2H), 8.09 (dd, J=8.3, 1.7 Hz, 1H), 7.48~7.37 (m, 3H), 3.32~3.23 (m, 1H), 2.92 (dd, J=15.5, 6.8 Hz, 1H), 2.70 (dd, J=15.5, 7.6 Hz, 1H), 1.38 (d, J=7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 166.89, 148.24, 138.07, 136.62, 133.80, 127.95, 127.36, 122.29, 122.13, 121.78, 116.90, 41.28, 21.89, 17.78; HRMS (ESI-TOF) calcd for C14H14N3O [M+H]+ 240.1104, found 240.1101.
3-氰基-2-(环丙基甲基)-N-(喹啉-8-基)丁酰胺(3b): 黄色油状液体. 1H NMR (400 MHz, CDCl3) δ: 9.88 (s, 1H), 8.74~8.55 (m, 2H), 8.01 (dd, J=8.3, 1.7 Hz, 1H), 7.44~7.24 (m, 3H), 3.06~2.86 (m, 1H), 2.57 (td, J=10.2, 4.2 Hz, 1H), 1.70 (d, J=6.9 Hz, 2H), 1.23 (d, J=7.0 Hz, 3H), 0.71~0.53 (m, 1H), 0.36~0.18 (m, 2H), -0.00 (tdd, J=9.2, 8.2, 4.3 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ: 170.46, 148.32, 136.42, 133.95, 127.94, 127.40, 122.00, 121.71, 116.91, 51.48, 35.38, 27.85, 15.55, 9.04, 5.01, 4.39; HRMS (ESI-TOF) calcd for C18H20N3O [M+H]+ 294.1504, found 294.1506.
2-(1-氰乙基)-N-(喹啉-8-基)戊-4-烯酰胺(3c): 黄色油状液体. 1H NMR (400 MHz, CDCl3) δ: 9.96 (s, 1H), 8.90~8.73 (m, 2H), 8.17 (dd, J=8.3, 1.7 Hz, 1H), 7.62~7.40 (m, 3H), 5.94~5.74 (m, 1H), 5.36~5.02 (m, 2H), 3.12 (dd, J=7.2, 6.0 Hz, 1H), 2.81~2.66 (m, 2H), 2.66~2.55 (m, 1H), 1.46 (d, J=7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 169.52, 148.32, 138.39, 136.42, 133.82, 133.48, 127.93, 127.36, 122.10, 121.74, 121.12, 119.01, 116.94, 50.37, 34.47, 27.50, 15.45; HRMS (ESI-TOF) calcd for C17H18N3O [M+H]+ 280.1504, found 280.1506.
3-氰基-2-甲基-N-(喹啉-8-基)丁酰胺(3d): 黄色油状液体. 1H NMR (400 MHz, CDCl3) δ: 10.03 (s, 1H), 8.88~8.69 (m, 2H), 8.18 (dd, J=8.3, 1.7 Hz, 1H), 7.66~7.40 (m, 3H), 3.22~3.16 (m, 1H), 2.97~2.89 (m, 1H), 1.52 (d, J=7.1 Hz, 3H), 1.43 (d, J=7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 172.60, 148.19, 137.99, 136.41, 134.48, 127.96, 127.42, 121.56, 121.52, 117.29, 116.48, 47.00, 16.97; HRMS (ESI-TOF) calcd for C15H16N3O [M+H]+ 254.1201, found 254.1202.
3-氰基-2-乙基-N-(喹啉-8-基)丁酰胺(3e): 黄色油状液体. 1H NMR (400 MHz, CDCl3) δ: 9.98 (s, 1H), 8.93~8.73 (m, 2H), 8.17 (dd, J=8.3, 1.7 Hz, 1H), 7.62~7.37 (m, 3H), 3.14~3.04 (m, 1H), 2.63 (ddd, J=9.4, 6.8, 5.0 Hz, 1H), 2.03~1.93 (m, 1H), 1.86 (dtd, J=13.8, 7.3, 5.1 Hz, 1H), 1.45 (d, J=7.2 Hz, 3H), 1.08 (s, 3H); 13C NMR (101 MHz, CDCl3) δ: 170.29, 148.35, 138.39, 136.44, 133.85, 127.93, 127.35, 122.08, 121.73, 121.40, 116.93, 52.45, 27.80, 23.45, 15.44, 11.74; HRMS (ESI- TOF) calcd for C16H18N3O [M+H]+ 268.1404, found 268.1406.
2-(1-氰乙基)-N-(喹啉-8-基)庚-6-烯酰胺(3f): 黄色油状液体. 1H NMR (400 MHz, CDCl3) δ: 9.99 (s, 1H), 8.95~8.71 (m, 2H), 8.17 (dd, J=8.3, 1.7 Hz, 1H), 7.63~7.40 (m, 3H), 5.88~5.70 (m, 1H), 5.06~4.89 (m, 2H), 3.08 (p, J=7.1 Hz, 1H), 2.69 (ddd, J=9.9, 6.6, 4.7 Hz, 1H), 2.12 (t, J=7.1 Hz, 2H), 2.02~1.92 (m, 1H), 1.82~1.73 (m, 1H), 1.57 (ddd, J=7.4, 4.5, 2.0 Hz, 2H), 1.44 (d, J=7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 170.22, 148.36, 138.39, 137.80, 136.43, 133.82, 127.93, 127.34, 122.11, 121.76, 121.34, 116.91, 115.32, 50.83, 33.52, 29.62, 28.16, 26.46, 15.45; HRMS (ESI-TOF) calcd for C17H18N3O [M+H]+ 280.1404, found 280.1402.
3-氰基-2-环己基-N-(喹啉-8-基)丁酰胺(3g): 黄色油状液体. 1H NMR (400 MHz, Chloroform-d) δ: 9.91 (s, 1H), 8.96~8.77 (m, 2H), 8.18 (dd, J=8.3, 1.7 Hz, 1H), 7.56~7.39 (m, 3H), 3.17 (dd, J=7.2, 5.8 Hz, 1H), 2.34 (dd, J=9.1, 5.8 Hz, 1H), 2.02 (ddd, J=12.1, 9.0, 3.8 Hz, 2H), 1.81 (t, J=9.6 Hz, 2H), 1.69 (q, J=5.9 Hz, 2H), 1.46~1.03 (m, 10H); 13C NMR (101 MHz, Chloroform-d) δ: 169.99, 148.34, 136.42, 133.77, 127.94, 127.42, 122.00, 121.70, 116.93, 57.03, 38.36, 30.99, 30.69, 26.12, 26.06, 25.86, 25.40, 16.14; HEMS (ESI-TOF) calcd for C20H24N3O [M+H]+ 322.1804, found 422.1802.
2-(1-氰乙基)-N-(喹啉-8-基)壬酰胺(3h): 黄色油状液体. 1H NMR (400 MHz, CDCl3) δ: 9.98 (s, 1H), 8.84~8.80 (m, 2H), 8.17 (dd, J=8.3, 1.7 Hz, 1H), 7.55~7.53 (m, 2H), 7.47 (dd, J=8.3, 4.2 Hz, 1H), 3.12~3.04 (m, 1H), 2.68 (ddd, J=9.7, 6.6, 4.8 Hz, 1H), 1.94 (d, J=5.8 Hz, 1H), 1.79~1.70 (m, 1H), 1.45 (d, J=7.2 Hz, 4H), 1.39~1.31 (m, 2H), 1.31~1.20 (m, 7H), 0.85~0.81 (m, 3H); 13C NMR (101 MHz, CDCl3) δ: 170.40, 148.33, 138.39, 136.42, 133.86, 127.93, 127.35, 122.05, 121.74, 121.41, 116.89, 50.94, 31.73, 30.26, 29.48, 29.02, 28.14, 27.28, 22.57, 15.47, 14.04; HRMS (ESI-TOF) calcd for C21H28N3O [M+H]+ 338.2204, found 338.2200.
3-氰基-2-(4-甲基苄基)-N-(喹啉-8-基)丁酰胺(3i): 黄色油状液体. 1H NMR (400 MHz, CDCl3) δ: 9.76 (s, 1H), 8.90~8.65 (m, 2H), 8.15 (dd, J=8.3, 1.7 Hz, 1H), 7.55~7.36 (m, 3H), 7.20 (d, J=8.0 Hz, 2H), 7.06 (d, J=7.7 Hz, 2H), 3.24 (dd, J=13.8, 8.2 Hz, 1H), 3.11 (dd, J=13.8, 7.0 Hz, 1H), 3.00 (dd, J=7.2, 5.6 Hz, 1H), 2.89 (ddd, J=8.2, 7.0, 5.5 Hz, 1H), 2.24 (s, 3H), 1.47 (d, J=7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 169.47, 148.14, 136.57, 136.32, 134.43, 133.80, 129.52, 128.84, 127.84, 127.33, 122.01, 121.63, 116.91, 52.83, 36.30, 27.54, 20.99, 15.78; HRMS (ESI-TOF) calcd for C22H22N3O [M+H]+ 344.1701, found 344.1703.
3-氰基-2-苯乙基-N-(喹啉-8-基)丁酰胺(3j): 黄色油状液体. 1H NMR (400 MHz, CDCl3) δ: 9.99 (s, 1H), 8.90~8.74 (m, 2H), 8.19 (dd, J=8.3, 1.7 Hz, 1H), 7.60~7.45 (m, 3H), 7.31~7.20 (m, 5H), 3.06 (p, J=7.1 Hz, 1H), 2.88~2.82 (m, 1H), 2.74~2.63 (m, 2H), 2.34 (dddd, J=13.8, 10.3, 8.8, 5.2 Hz, 1H), 2.06 (dddd, J=13.5, 9.2, 7.6, 4.2 Hz, 1H), 1.42 (d, J=7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 169.99, 148.36, 140.51, 138.42, 136.44, 133.80, 128.64, 128.51, 127.96, 127.36, 126.34, 122.21, 121.80, 121.18, 117.00, 49.96, 33.24, 31.82, 28.36, 15.43; HRMS (ESI-TOF) calcd for C22H22N3O [M+H]+ 294.1504, found 294.1506.
3-氰基-2-(4-氟苄基)-N-(喹啉-8-基)丁酰胺(3k): 黄色油状液体. 1H NMR (400 MHz, CDCl3) δ: 9.72 (s, 1H), 8.80~8.72 (m, 2H), 8.17~8.13 (m, 1H), 7.54~7.51 (m, 2H), 7.44 (dd, J=8.4, 4.4 Hz, 1H), 7.29~7.25 (m, 2H), 6.97~6.89 (m, 2H), 3.24 (dd, J=13.8, 9.0 Hz, 1H), 3.10 (dd, J=13.8, 6.3 Hz, 1H), 3.03 (dd, J=7.1, 5.6 Hz, 1H), 2.93~2.87 (m, 1H), 1.50~1.48 (m, 3H); 13C NMR (101 MHz, CDCl3) δ: 169.17, 148.30, 148.16, 136.52, 136.46, 133.57, 133.31, 133.27, 130.56, 130.48, 127.86, 127.35, 127.31, 122.18, 122.11, 121.79, 121.71, 120.91, 116.99, 116.81, 115.79, 115.58, 52.82, 41.32, 35.83, 27.76, 21.89, 17.80, 15.66; HRMS (ESI-TOF) calcd for C21H19N3O [M+H]+ 348.3911, found 349.3907.
3-氰基-2-(环丁基甲基)-N-(喹啉-8-基)-丁酰胺(3l): 黄色油状液体. 1H NMR (400 MHz, CDCl3) δ: 9.93 (s, 1H), 8.92~8.71 (m, 2H), 8.18 (dd, J=8.3, 1.7 Hz, 1H), 7.66~7.48 (m, 3H), 3.12~2.95 (m, 1H), 2.60 (ddd, J=10.1, 6.6, 4.6 Hz, 1H), 2.43 (qd, J=8.2, 6.5 Hz, 1H), 2.24~2.14 (m, 1H), 2.13~1.99 (m, 2H), 1.86~1.66 (m, 5H), 1.44 (d, J=7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 172.17, 148.24, 138.56, 137.11, 136.32, 134.53, 127.95, 127.42, 121.56, 121.46, 117.68, 116.41, 51.72, 39.21, 33.94, 28.52, 28.25, 18.48; HRMS (ESI-TOF) calcd for C19H22N3O [M+H]+ 308.1504, found 308.1506.
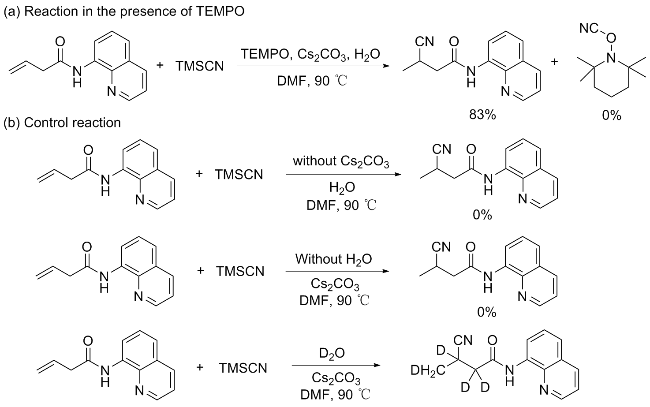
3-氰基-2,2,3,4-四氘代-N-(8-氨基喹啉)丁酰胺(4): 1H NMR (400 MHz, CDCl3) δ: 9.87 (s, 1H), 8.90~8.66 (m, 2H), 8.14 (dd, J=8.3, 1.7 Hz, 1H), 7.54~7.41 (m, 3H), 1.45~1.41 (m, 2H).