Chinese Journal of Organic Chemistry ›› 2019, Vol. 39 ›› Issue (11): 3169-3175.DOI: 10.6023/cjoc201904028 Previous Articles Next Articles
收稿日期:2019-04-10
发布日期:2019-07-09
通讯作者:
阿布拉江·克依木
E-mail:ablajan209@hotmail.com
基金资助:
Liang Jie, Ma Huifang, Ablajan Keyume*( )
)
Received:2019-04-10
Published:2019-07-09
Contact:
Ablajan Keyume
E-mail:ablajan209@hotmail.com
Supported by:Share
Liang Jie, Ma Huifang, Ablajan Keyume. High-Selective One-Pot Synthesis of Spirocyclopropane Pyrazolones Promoted by 4-Dimethylaminopyridine[J]. Chinese Journal of Organic Chemistry, 2019, 39(11): 3169-3175.
 | |||||
| Entry | Base | Solvent | Temp./℃ | Yieldb/% | transc/cis |
| 1 | No base | CH3CN | 81 | NR | NR |
| 2 | NaOH | CH3CN | 81 | 41 | 45:55 |
| 3 | Cs2CO3 | CH3CN | 81 | 48 | 48:52 |
| 4 | Pyridine | CH3CN | 81 | 82c | 72:28 |
| 5 | TEBA | CH3CN | 81 | 56 | 80:20 |
| 6 | CTAB | CH3CN | 81 | 60 | 85:15 |
| 7 | DABCO | CH3CN | 81 | 70 | 72:28 |
| 8 | DMAP | CH3CN | 81 | 86 | 94:6 |
| 9 | DMAP | CH2Cl2 | 81 | 65 | 40:60 |
| 10 | DMAP | H2O | 100 | Trace | NR |
| 11 | DMAP | CH3CO2Et | 81 | 78 | 88:12 |
| 12 | DMAP | CH3CN | 81 | 86d | 94:6 |
| 13 | DMAP | CH3CN | 25 | 36 | 46:54 |
| 14 | DMAP | CH3CN | 60 | 68 | 79:21 |
| 15 | DMAP | CH3CN | 81 | 50e | 90:10 |
 | |||||
| Entry | Base | Solvent | Temp./℃ | Yieldb/% | transc/cis |
| 1 | No base | CH3CN | 81 | NR | NR |
| 2 | NaOH | CH3CN | 81 | 41 | 45:55 |
| 3 | Cs2CO3 | CH3CN | 81 | 48 | 48:52 |
| 4 | Pyridine | CH3CN | 81 | 82c | 72:28 |
| 5 | TEBA | CH3CN | 81 | 56 | 80:20 |
| 6 | CTAB | CH3CN | 81 | 60 | 85:15 |
| 7 | DABCO | CH3CN | 81 | 70 | 72:28 |
| 8 | DMAP | CH3CN | 81 | 86 | 94:6 |
| 9 | DMAP | CH2Cl2 | 81 | 65 | 40:60 |
| 10 | DMAP | H2O | 100 | Trace | NR |
| 11 | DMAP | CH3CO2Et | 81 | 78 | 88:12 |
| 12 | DMAP | CH3CN | 81 | 86d | 94:6 |
| 13 | DMAP | CH3CN | 25 | 36 | 46:54 |
| 14 | DMAP | CH3CN | 60 | 68 | 79:21 |
| 15 | DMAP | CH3CN | 81 | 50e | 90:10 |
 | ||||||
| Entry | R1 | R2 | R3 | 4 | Yieldb/% | trans/cisc |
| 1 | Ph | 4-ClC6H4 | Me | 4a | 62 | 6:1 |
| 2 | Ph | 4-BrC6H4 | Me | 4b | 67 | 8:1 |
| 3 | Ph | 2-O2NC6H4 | Me | 4c | 84 | 11:1 |
| 4 | Ph | 2-ClC6H4 | Me | 4d | 85 | 12:1 |
| 5 | Ph | 2, 4-Cl2C6H4 | Me | 4e | 79 | 10:1 |
| 6 | 4-CH3C6H4 | 4-ClC6H4 | Me | 4f | 62 | 15:1 |
| 7 | 4-CH3C6H4 | 4-BrC6H4 | Me | 4g | 65 | 11:1 |
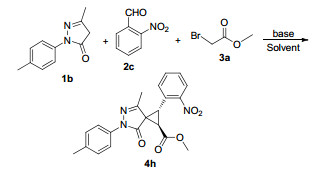
| 8 | 4-CH3C6H4 | 2-O2NC6H4 | Me | 4h | 86 | 18:1 |
| 9 | 4-CH3C6H4 | 2-ClC6H4 | Me | 4i | 86 | 17:1 |
| 10 | 4-CH3C6H4 | 2, 4-Cl2C6H4 | Me | 4j | 80 | 15:1 |
| 11 | 4-CH3C6H4 | Ph | Me | 4k | 65 | 6:1 |
| 12 | 4-ClC6H4 | 4-ClC6H4 | Me | 4l | 62 | 9:1 |
| 13 | 4-ClC6H4 | 4-BrC6H4 | Me | 4m | 66 | 8:1 |
| 14 | 4-ClC6H4 | 2-O2NC6H4 | Me | 4n | 58 | > 25:1 |
| 15 | 4-ClC6H4 | 2, 4-Cl2C6H4 | Me | 4o | 82 | 15:1 |
| 16 | 2-ClC6H4 | 4-ClC6H4 | Me | 4p | 77 | 11:1 |
| 17 | 2-ClC6H4 | 4-BrC6H4 | Me | 4q | 78 | 12:1 |
| 18 | 2-ClC6H4 | 2-O2NC6H4 | Me | 4r | 83 | 18:1 |
| 19 | 2-ClC6H4 | 2, 4-Cl2C6H4 | Me | 4s | 80 | 13:1 |
| 20 | Ph | 2, 4-Cl2C6H4 | C(Me)3 | 4t | 89 | > 25:1 |
| 21 | 4-CH3C6H4 | 2-O2NC6H4 | C(Me)3 | 4u | 87 | > 25:1 |
| 22 | 2-ClC6H4 | 2-O2NC6H4 | C(Me)3 | 4v | 87 | > 25:1 |
| 23 | 4-ClC6H4 | 2, 4-Cl2C6H4 | C(Me)3 | 4w | 85 | > 25:1 |
 | ||||||
| Entry | R1 | R2 | R3 | 4 | Yieldb/% | trans/cisc |
| 1 | Ph | 4-ClC6H4 | Me | 4a | 62 | 6:1 |
| 2 | Ph | 4-BrC6H4 | Me | 4b | 67 | 8:1 |
| 3 | Ph | 2-O2NC6H4 | Me | 4c | 84 | 11:1 |
| 4 | Ph | 2-ClC6H4 | Me | 4d | 85 | 12:1 |
| 5 | Ph | 2, 4-Cl2C6H4 | Me | 4e | 79 | 10:1 |
| 6 | 4-CH3C6H4 | 4-ClC6H4 | Me | 4f | 62 | 15:1 |
| 7 | 4-CH3C6H4 | 4-BrC6H4 | Me | 4g | 65 | 11:1 |
| 8 | 4-CH3C6H4 | 2-O2NC6H4 | Me | 4h | 86 | 18:1 |
| 9 | 4-CH3C6H4 | 2-ClC6H4 | Me | 4i | 86 | 17:1 |
| 10 | 4-CH3C6H4 | 2, 4-Cl2C6H4 | Me | 4j | 80 | 15:1 |
| 11 | 4-CH3C6H4 | Ph | Me | 4k | 65 | 6:1 |
| 12 | 4-ClC6H4 | 4-ClC6H4 | Me | 4l | 62 | 9:1 |
| 13 | 4-ClC6H4 | 4-BrC6H4 | Me | 4m | 66 | 8:1 |
| 14 | 4-ClC6H4 | 2-O2NC6H4 | Me | 4n | 58 | > 25:1 |
| 15 | 4-ClC6H4 | 2, 4-Cl2C6H4 | Me | 4o | 82 | 15:1 |
| 16 | 2-ClC6H4 | 4-ClC6H4 | Me | 4p | 77 | 11:1 |
| 17 | 2-ClC6H4 | 4-BrC6H4 | Me | 4q | 78 | 12:1 |
| 18 | 2-ClC6H4 | 2-O2NC6H4 | Me | 4r | 83 | 18:1 |
| 19 | 2-ClC6H4 | 2, 4-Cl2C6H4 | Me | 4s | 80 | 13:1 |
| 20 | Ph | 2, 4-Cl2C6H4 | C(Me)3 | 4t | 89 | > 25:1 |
| 21 | 4-CH3C6H4 | 2-O2NC6H4 | C(Me)3 | 4u | 87 | > 25:1 |
| 22 | 2-ClC6H4 | 2-O2NC6H4 | C(Me)3 | 4v | 87 | > 25:1 |
| 23 | 4-ClC6H4 | 2, 4-Cl2C6H4 | C(Me)3 | 4w | 85 | > 25:1 |
| [1] |
(a) Kinder, F. R. J.; Wang, R.-M.; Bauta, W. E.; Bair, K. W. M. Bioorg. Med. Chem. Lett. 1996, 6, 1029.
doi: 10.1016/0960-894X(96)00167-9 |
|
(b) Wessjohann, L. A.; Brandt, W. Chem. Rev. 2003, 103, 1625.
doi: 10.1016/0960-894X(96)00167-9 |
|
|
(c) Chen, D. Y.-K.; Pouwer, R. H.; Richard, J.-A. Chem. Soc. Rev. 2012, 41, 4631.
doi: 10.1016/0960-894X(96)00167-9 |
|
|
(d) Djerassi, C.; Doss, G. A. New J. Chem. 1990, 14, 713.
doi: 10.1016/0960-894X(96)00167-9 |
|
|
(e) Donaldson, W. A. Tetrahedron 2001, 57, 8589.
doi: 10.1016/0960-894X(96)00167-9 |
|
|
(f) Faust, R. Angew. Chem. 2001, 113, 2312.
doi: 10.1016/0960-894X(96)00167-9 |
|
|
(g) Qian, P.; Du, B. G.; Song, R. C.; Wu, X. D.; Mei, H. B.; Han, J. L.; Pan, Y. J. Org. Chem. 2016, 81, 6546.
doi: 10.1016/0960-894X(96)00167-9 |
|
| [2] |
Sampson P. B. Liu Y. Patel N. K. Feher M. Forrest B. J. Med. Chem. 2015 58 130.
doi: 10.1021/jm500537u |
| [3] |
Sahlberg C. Engelhardt P. J. Med. Chem. 1999 42 4150.
doi: 10.1021/jm990095j |
| [4] |
McMorris T. C. Kelner M. J. Wang W. Yu J. Estes L. A. Taetle R. J. Nat. Prod. 1996 59 896.
doi: 10.1021/np960450y |
| [5] |
(a) Cordero, F. M.; Pisaneschi, F.; Salvati, M.; Paschetta, V.; Ollivier, J.; Salaun, J.; Brandi, A. J. Org. Chem. 2003, 68, 3271.
doi: 10.1021/jo034003g |
|
(b) Basavaiah, D.; Rao, A. J.; Satyanarayana, T. Chem. Rev. 2003, 103, 811.
doi: 10.1021/jo034003g |
|
| [6] |
(a) Lebel, H.; Marcoux, J. F.; Molinaro, C.; Charette, A. B. Chem. Rev. 2003, 103, 977.
doi: 10.1021/cr010007e |
|
(b) Kulinkovich, O. G.; Meijere, D. A. Chem. Rev. 2000, 100, 2789.
doi: 10.1021/cr010007e |
|
|
(c) Mukherjee, P.; Das, A. R. J. Org. Chem. 2017, 82, 2794.
doi: 10.1021/cr010007e |
|
| [7] |
Papageorgiou C. D. Cubillo de Dios M. A. Ley S. V. Gaunt M. J. Angew. Chem. Int. Ed. 2004 43 4641.
doi: 10.1002/anie.200460234 |
| [8] |
(a) Sun, X. L.; Tang, Y. Acc. Chem. Res. 2008, 41, 937.
doi: 10.1021/ar800108z |
|
(b) Kakei, H.; Sone, T.; Sohtome, Y.; Matsunaga, S.; Shibasaki, M. J. Am. Chem. Soc. 2007, 129, 13410.
doi: 10.1021/ar800108z |
|
|
(c) Wang, J.; Liu, X. H.; Dong, S. X.; Lin, L. L.; Feng, X. M. J. Org. Chem. 2013, 78, 6322.
doi: 10.1021/ar800108z |
|
|
(d) Guo, J.; Liu, Y. B.; Li, X. Q.; Liu, X. H.; Lin, L. L.; Feng, X. M. Chem. Sci. 2016, 7, 2717.
doi: 10.1021/ar800108z |
|
| [9] |
(a) Sawada, T.; Nakada, M. Org. Lett. 2013, 15, 1004.
doi: 10.1021/ol303459x |
|
(b) Lindsay; V. N. G.; Nicolas, C.; Charette, A. B. J. Am. Chem. Soc. 2011, 133, 8972.
doi: 10.1021/ol303459x |
|
|
(c) Xu, X.; Zhu, S.; Cui, X.; Wojtas, L.; Zhang, X. P. Angew. Chem. 2013, 125, 12073.
doi: 10.1021/ol303459x |
|
|
(d) Xu, Z.-H.; Zhu, S.-N.; Sun, X.-L.; Tang, Y.; Dai, L.-X. Chem. Commun. 2007, 38, 1960.
doi: 10.1021/ol303459x |
|
| [10] |
(a) Arai, S.; Nakayama, K.; Hatano, K.; Shioiri, T. J. Org. Chem. 1998, 63, 9572.
doi: 10.1021/jo981409y |
|
(b) Miyagawa, T.; Tatenuma, T.; Tadokoro, M.; Satoh, T. Tetrahedron, 2008, 64, 5279.
doi: 10.1021/jo981409y |
|
| [11] |
(a) Newcomb, E. T.; Ferreira, E. M. Org. Lett. 2013, 15, 1772.
doi: 10.1021/ol400625f |
|
(b) Robinson, A.; Aggarwal, V. K. Angew. Chem. 2010, 122, 6823.
doi: 10.1021/ol400625f |
|
| [12] | Yuan Z.-B. Fang X.-X. Li X.-Y. Wu J. Yao H.-Q. Lin A.-J. J. Org. Chem. 2015 80 1112. |
| [13] |
Pyne S. G. Dong Z. Skelton B. W. White A. H. J. Org. Chem. 1997 62 2337.
doi: 10.1021/jo962216i |
| [14] |
Hanessian S. Andreotti D. Gomtsyan A. J. Am. Chem. Soc. 1995 117 10393.
doi: 10.1021/ja00146a029 |
| [15] |
Kimber M. C. Taylor D. K. J. Org. Chem. 2002 67 3142.
doi: 10.1021/jo0110496 |
| [16] | Avery T. D. Jenkins N. F. Kimber M. C. Lupton D. W. Taylor D. K. Chem. Commun. 2002 33 28. |
| [17] |
Wang Q. Song X. K. Chen J. Yan C. G. J. Comb. Chem. 2009 11 1007.
doi: 10.1021/cc900005v |
| [18] | Ošeka M. Noole A. Žari S. Öeren M. Järving I. Lopp M. Kanger T. Eur. J. Org. Chem. 2014 17 3599. |
| [19] |
Ren Z. J. Cao W. G. Tong W. Q. Chen J. Deng H. M. Wu D. Y. Synth. Commun. 2008 38 2200.
doi: 10.1080/00397910802029406 |
| [20] |
Ren Z. J. Cao W. G. Chen J. Chen Y. L. Deng H. M. Shao M. Wu D. Y. Tetrahedron 2008 64 5156.
doi: 10.1016/j.tet.2008.03.049 |
| [21] |
Li J. H. Feng T. F. Du D. M. J. Org. Chem. 2015 80 11369.
doi: 10.1021/acs.joc.5b01940 |
| [22] |
(a) Ablajan, K.; Zeynepgul, E.; Wang, L. J.; Feng, J. Tetrahedron 2014, 70, 3976.
doi: 10.1016/j.tet.2014.04.088 |
|
(b) Wang, L. J.; Ablajan, K.; Feng, J. Ultrason. Sonochem. 2015, 22, 113.
doi: 10.1016/j.tet.2014.04.088 |
|
|
(c) Li, W. B.; Reyhangul, R.; Ablajan, K.; Zulpiya, G. Tetrahedron 2017, 73, 164.
doi: 10.1016/j.tet.2014.04.088 |
|
| [23] |
(a) Khan, A.; Lal, M.; Sidick Basha, R. Synthesis 2013, 45, 406.
doi: 10.1055/s-00000084 |
|
(b) Wang, Q.-F.; Hou, H.; Hui, L.; Yan, C.-G. J. Org. Chem. 2009, 74, 7403.
doi: 10.1055/s-00000084 |
|
|
(c) Chuang, C.-P.; Chen, K.-P. Tetrahedron 2012, 68, 1401.
doi: 10.1055/s-00000084 |
| [1] | Xiaoping Xu, Yifei Zhang, Xiaoyu Mo, Jun Jiang. Rh-Catalyzed C—H Functionalization Reaction between 3-Diazoindolin-2-imines and Pyrazolones for the Construction of 3-Pyrazolyl Indoles [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2519-2527. |
| [2] | Wensheng Zhang, Yan Li, Haiyan Cui, Xiaoli Su, Supeng Xu. One-Pot Synthesis of N-Substituted Isoindolin-1-ones via Reductive Amination/Lactamization of Methyl 2-Formylbenzoate [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2456-2461. |
| [3] | Xianghao Luo, Yibi Xie, Nianyu Huang, Long Wang. Ugi Four-Component Reaction Based on in-situ Capture of Isocyanide and Post-Modification Tandem Reaction: One-Pot Synthesis of Nitrogen Heterocycles [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 838-846. |
| [4] | Jinni Liu, Yibi Xie, Qingqing Yang, Nianyu Huang, Long Wang. Ugi Four-Component Reaction Based on the in situ Capture of Amines and Subsequent Modification Tandem Cyclization Reaction: "One-Pot" Synthesis of Six- and Seven-Membered Heterocycles [J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2374-2383. |
| [5] | He Shuwang, Yan Shiqiang, Guo Wei, Zhai Guangxi, Zhang Wei. One-Pot Synthesis of Amino Alcohols from Styrenes [J]. Chinese Journal of Organic Chemistry, 2020, 40(7): 2094-2098. |
| [6] | Lan Jin, Xie Zongbo, Yang Jiangnan, Meng Jia, Liu Yishuai, Le Zhanggao. One-Pot Multi-component Synthesis of N,O-Acetal Compounds Catalyzed by D-Proline at Room Temperature [J]. Chinese Journal of Organic Chemistry, 2020, 40(5): 1331-1336. |
| [7] | Yan Lijun, Yan Yuxin, Chen Xuebing, Wang Yongchao. Advances in Multicomponent Asymmetric Cascade Synthesis Involving Hydrogen-Bond-Activated Nitroolefins [J]. Chinese Journal of Organic Chemistry, 2020, 40(4): 856-872. |
| [8] | Yan Lijun, Xu Han, Wang Yan, Dong Jianwei, Wang Yongchao. Advances in Multicomponent Asymmetric Cascade Synthesis Involving Nitroolefin Catalyzed by Diarylprolinol Derivatives [J]. Chinese Journal of Organic Chemistry, 2020, 40(2): 284-299. |
| [9] | Wan Linxi, Gao Feng, Chen Wei, Zhou Xianli. One-Pot Synthesis of 2-((2-Aminobenzoyl)amino)benzoic Acid Derivatives [J]. Chin. J. Org. Chem., 2019, 39(8): 2270-2276. |
| [10] | Xu Jing, Fan Weigang, Popowycz Florence, Queneau Yves, Gu Yanlong. Multicomponent Reactions: A New Strategy for Enriching the Routes of Value-Added Conversions of Bio-platform Molecules [J]. Chin. J. Org. Chem., 2019, 39(8): 2131-2138. |
| [11] | Liu Xiaoqin, Wang Fei, Sun Hui, Zheng Mengya, Wang Hualan, Gong Kai. Synthesis of Tetrahydrobenzo[a]xanthen-11-ones Catalyzed byAcid Ionic Liquid Functionalized β-Cyclodextrin in Water [J]. Chinese Journal of Organic Chemistry, 2019, 39(10): 2843-2850. |
| [12] | Sun Xiaoli, Sun Zhenhua, Han Minmin, Han Jing, He Weimin, Chen Jie, Deng Hongmei, Shao Min, Zhang Hui, Cao Weiguo. Synthesis of Perfluoroalkylated Fluorenes with Phosphonate Group via One-Pot Synthesis Process [J]. Chin. J. Org. Chem., 2019, 39(1): 192-199. |
| [13] | Bai Feicheng, Hu Deqing, Liu Yunyun, Wei Li. One-Pot and Multicomponent Synthesis of N-Substituted-4-hydroxyl-2-pyridones [J]. Chin. J. Org. Chem., 2018, 38(8): 2054-2059. |
| [14] | Yang Zhonghua, Liu Lanye, Zhao Yihe, Hong Yuanlin, Ruan Hongli, Lü Chengwei. Choline Chloride as Catalyst towards the Attractive Yonemitsu Reaction of Benzaldehyde, Indole, and Malononitrile [J]. Chin. J. Org. Chem., 2018, 38(10): 2761-2766. |
| [15] | Qian Cunwei, Zang Shiyu, Zhou Qian, Wang Dong, Li Wanxin, Wang Maoyuan. One-Pot Synthesis and Optical Properties of 2,5-Diphenylthiophene Derivatives [J]. Chin. J. Org. Chem., 2017, 37(7): 1781-1786. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||