Chinese Journal of Organic Chemistry ›› 2023, Vol. 43 ›› Issue (12): 4227-4238.DOI: 10.6023/cjoc202304003 Previous Articles Next Articles
ARTICLES
唐朵朵, 黄丹凤*( ), 王克虎, 马虎, 冯杨, 任园园, 王君姣, 胡雨来
), 王克虎, 马虎, 冯杨, 任园园, 王君姣, 胡雨来
收稿日期:2023-04-04
修回日期:2023-06-05
发布日期:2023-08-16
基金资助:
Duoduo Tang, Danfeng Huang*( ), Kehu Wang, Hu Ma, Yang Feng, Yuanyuan Ren, Junjiao Wang, Yulai Hu
), Kehu Wang, Hu Ma, Yang Feng, Yuanyuan Ren, Junjiao Wang, Yulai Hu
Received:2023-04-04
Revised:2023-06-05
Published:2023-08-16
Contact:
*E-mail: Supported by:Share
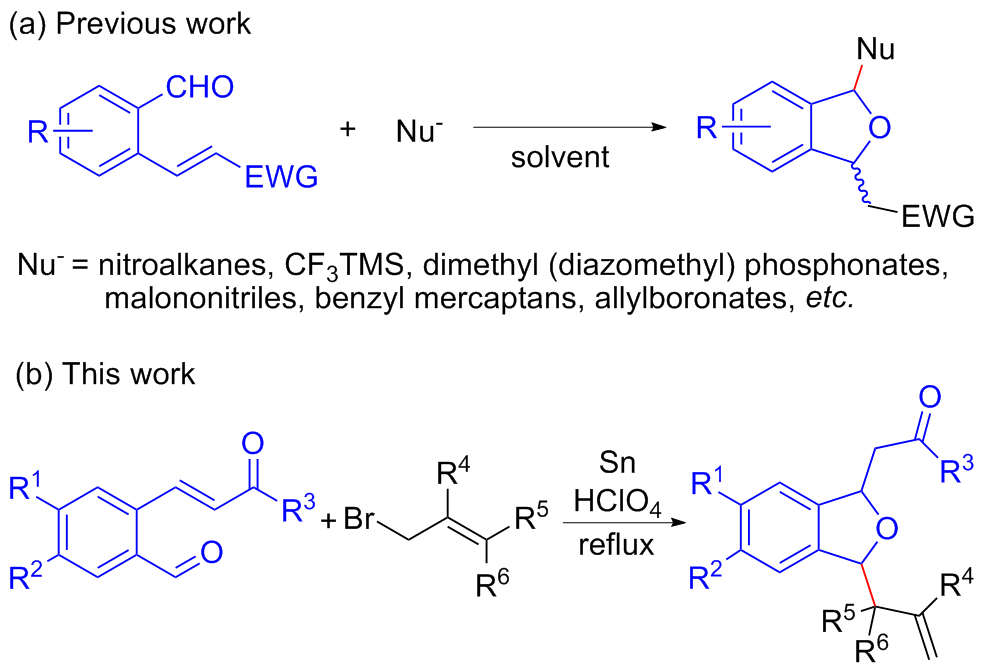
Duoduo Tang, Danfeng Huang, Kehu Wang, Hu Ma, Yang Feng, Yuanyuan Ren, Junjiao Wang, Yulai Hu. Tin Powder-Promoted Synthesis of 1,3-Disubstituted 1,3-Dihydroisobenzofuran Compounds[J]. Chinese Journal of Organic Chemistry, 2023, 43(12): 4227-4238.
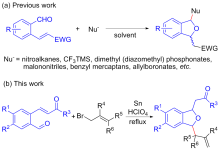
| Entry | Molar ratio of 1a∶2a∶Sn | Additive (equiv.) | Solvent | Timeb/h | Yieldc/% | cis∶transd[ |
|---|---|---|---|---|---|---|
| 1 | 1.0∶2.0∶2.5 | None | THF | 12 | 66 | 1.0∶2.4 |
| 2 | 1.0∶3.0∶3.5 | None | THF | 12 | 56 | 1.0∶1.0 |
| 3 | 1.0∶1.0∶1.5 | None | THF | 9 | 54 | 1.0∶1.2 |
| 4 | 1.0∶2.0∶2.5 | In(OTf)3 (0.2) | THF | 6 | 50 | 2.2∶1.0 |
| 5 | 1.0∶2.0∶2.5 | AgOTf (0.2) | THF | 12 | 67 | 1.0∶2.0 |
| 6 | 1.0∶2.0∶2.5 | Fe(OTf)3 (0.2) | THF | 7 | 69 | 1.0∶1.5 |
| 7 | 1.0∶2.0∶2.5 | BF3•Et2O (0.2) | THF | 8 | 58 | 1.6∶1.0 |
| 8 | 1.0∶2.0∶2.5 | TsOH (0.2) | THF | 8 | 63 | 1.1∶1.0 |
| 9 | 1.0∶2.0∶2.5 | TfOH (0.2) | THF | 8 | 54 | 1.0∶1.0 |
| 10 | 1.0∶2.0∶2.5 | H3PO4 (0.2) | THF | 9 | 36 | 1.3∶1.0 |
| 11 | 1.0∶2.0∶2.5 | HCl (0.2) | THF | 9 | 67 | 1.0∶1.0 |
| 12 | 1.0∶2.0∶2.5 | HClO4 (0.2) | THF | 5 | 70 | 1.0∶1.2 |
| 13 | 1.0∶2.0∶2.5 | HClO4 (0.5) | THF | 4 | 78 | 1.4∶1.0 |
| 14 | 1.0∶2.0∶2.5 | HClO4 (1) | THF | 3 | 53 | 1.8∶1.0 |
| 15 | 1.0∶2.0∶2.5 | HClO4 (0.5) | Dioxane | 1 | 58 | 1.2∶1.0 |
| 16 | 1.0∶2.0∶2.5 | HClO4 (0.5) | DCM | 14 | 30 | 1.0∶1.8 |
| 17 | 1.0∶2.0∶2.5 | HClO4 (0.5) | Toluene | 1 | — | — |
| 18 | 1.0∶2.0∶2.5 | HClO4 (0.5) | CH3CN | 1 | 53 | 1.0∶1.0 |
| 19 | 1.0∶2.0∶2.5 | HClO4 (0.5) | EtOH | 1 | 83 | 1.0∶1.1 |
| Entry | Molar ratio of 1a∶2a∶Sn | Additive (equiv.) | Solvent | Timeb/h | Yieldc/% | cis∶transd[ |
|---|---|---|---|---|---|---|
| 1 | 1.0∶2.0∶2.5 | None | THF | 12 | 66 | 1.0∶2.4 |
| 2 | 1.0∶3.0∶3.5 | None | THF | 12 | 56 | 1.0∶1.0 |
| 3 | 1.0∶1.0∶1.5 | None | THF | 9 | 54 | 1.0∶1.2 |
| 4 | 1.0∶2.0∶2.5 | In(OTf)3 (0.2) | THF | 6 | 50 | 2.2∶1.0 |
| 5 | 1.0∶2.0∶2.5 | AgOTf (0.2) | THF | 12 | 67 | 1.0∶2.0 |
| 6 | 1.0∶2.0∶2.5 | Fe(OTf)3 (0.2) | THF | 7 | 69 | 1.0∶1.5 |
| 7 | 1.0∶2.0∶2.5 | BF3•Et2O (0.2) | THF | 8 | 58 | 1.6∶1.0 |
| 8 | 1.0∶2.0∶2.5 | TsOH (0.2) | THF | 8 | 63 | 1.1∶1.0 |
| 9 | 1.0∶2.0∶2.5 | TfOH (0.2) | THF | 8 | 54 | 1.0∶1.0 |
| 10 | 1.0∶2.0∶2.5 | H3PO4 (0.2) | THF | 9 | 36 | 1.3∶1.0 |
| 11 | 1.0∶2.0∶2.5 | HCl (0.2) | THF | 9 | 67 | 1.0∶1.0 |
| 12 | 1.0∶2.0∶2.5 | HClO4 (0.2) | THF | 5 | 70 | 1.0∶1.2 |
| 13 | 1.0∶2.0∶2.5 | HClO4 (0.5) | THF | 4 | 78 | 1.4∶1.0 |
| 14 | 1.0∶2.0∶2.5 | HClO4 (1) | THF | 3 | 53 | 1.8∶1.0 |
| 15 | 1.0∶2.0∶2.5 | HClO4 (0.5) | Dioxane | 1 | 58 | 1.2∶1.0 |
| 16 | 1.0∶2.0∶2.5 | HClO4 (0.5) | DCM | 14 | 30 | 1.0∶1.8 |
| 17 | 1.0∶2.0∶2.5 | HClO4 (0.5) | Toluene | 1 | — | — |
| 18 | 1.0∶2.0∶2.5 | HClO4 (0.5) | CH3CN | 1 | 53 | 1.0∶1.0 |
| 19 | 1.0∶2.0∶2.5 | HClO4 (0.5) | EtOH | 1 | 83 | 1.0∶1.1 |
| [1] |
Karmakar R.; Pahari P.; Mal D. Chem. Rev. 2014, 114, 6213.
doi: 10.1021/cr400524q pmid: 24823231 |
| [2] |
Lovey R. G.; Elliott A. J.; Kaminski J. J.; Loebenberg D.; Parme- giani R. M.; Rane D. F.; Girijavallabhan V. M.; Pike R. E.; Guzik H.; Antonacci B. J. Med. Chem. 1992, 35, 4221.
pmid: 1433223 |
| [3] |
Len C.; Selouane A.; Postel D.; Villa P.; Aubertin A.-M.; Egron D.; Gosselin G.; Périgaud C. Nucleos. Nucleot. Nucl. 2003, 22, 943.
doi: 10.1081/NCN-120022691 |
| [4] |
Coote S. J.; Davies S. G.; Middlemiss D.; Naylor A. J. Organomet. Chem. 1989, 379, 81.
doi: 10.1016/0022-328X(89)80027-0 |
| [5] |
Luzzio F. A.; Okoromoba O. E. Tetrahedron Lett. 2011, 52, 6530.
doi: 10.1016/j.tetlet.2011.09.123 |
| [6] |
Yuan H.; Gong Y. J. Fluorine Chem. 2013, 149, 125.
doi: 10.1016/j.jfluchem.2013.02.002 |
| [7] |
Wei X.; Chen G.; Peng Y. Tetrahedron Lett. 2020, 61, 152174.
doi: 10.1016/j.tetlet.2020.152174 |
| [8] |
Maity S.; Saha M.; Hazra G.; Ghorai P. Org. Lett. 2017, 19, 5872.
doi: 10.1021/acs.orglett.7b02862 |
| [9] |
Nath U.; Chowdhury D.; Pan S. C. Adv. Synth. Catal. 2018, 360, 1628.
doi: 10.1002/adsc.v360.8 |
| [10] |
Yang X.; Pang S.; Cheng F.; Zhang Y.; Lin Y.-W.; Yuan Q.; Zhang F.-L.; Huang Y.-Y. J. Org. Chem. 2017, 82, 10388.
doi: 10.1021/acs.joc.7b01856 |
| [11] |
Bao M.; Nakamura H.; Inoue A.; Yamamoto Y. Chem. Lett. 2002, 31, 158.
doi: 10.1246/cl.2002.158 |
| [12] |
Capriati V.; Florio S.; Luisi R.; Perna F. M.; Salomone A. J. Org. Chem. 2006, 71, 3984.
pmid: 16674080 |
| [13] |
Chao B.; Dittmer D. C. Tetrahedron Lett. 2000, 41, 6001.
doi: 10.1016/S0040-4039(00)01047-9 |
| [14] |
Jarrige L.; Carboni A.; Dagousset G.; Levitre G.; Magnier E.; Masson G. Org. Lett. 2016, 18, 2906.
doi: 10.1021/acs.orglett.6b01257 pmid: 27276522 |
| [15] |
Mukaiyama T.; Harada T. Chem. Lett. 1981, 10, 1527.
doi: 10.1246/cl.1981.1527 |
| [16] |
(a) Zhao P.; Huang D.; Wang F.; Han T.; Yang M.; Wang K. H.; Hu Y. Appl. Organomet. Chem. 2021, 36, e6479.
doi: 10.1002/aoc.v36.1 |
|
(b) Wang S.; Wang K. H.; Chang B.; Huang D.; Hu Y. Appl. Organomet. Chem. 2021, 35, e6249.
doi: 10.1002/aoc.v35.7 |
|
|
(c) Wang X.; Huang D.; Wang K. H.; Liu J.; Zong W.; Wang J.; Su Y.; Hu Y. Appl. Organomet. Chem. 2019, 33, e4995.
doi: 10.1002/aoc.v33.8 |
|
|
(d) Wang X.; Huang D.; Wang K. H.; Su Y.; Hu Y. J. Org. Chem. 2019, 84, 6946.
doi: 10.1021/acs.joc.9b00733 |
|
|
(e) Elaas N. A.; Elaas W. A.; Huang D.; Hu Y.; Wang K.-H. Curr. Org. Synth. 2017, 14, 1156.
|
|
| [17] |
Zhao Z.; Wang J.; Huang D.; Yang Z.; Zhao F.; Hu Y.; Xu W.; Hu Y. Chin. J. Org.Chem. 2020, 40, 2026. (in Chinese)
|
|
(赵转霞, 王君姣, 黄丹凤, 杨政, 赵芳霞, 虎永琴, 徐炜刚, 胡雨来, 有机化学, 2020, 40, 2026.)
doi: 10.6023/cjoc202003002 |
|
| [18] |
(a) Yamamoto Y.; Asao N. Chem. Rev. 1993, 93, 2207.
doi: 10.1021/cr00022a010 |
|
(b) Zha Z.; Qiao S.; Jiang J.; Wang Y.; Miao Q.; Wang Z. Tetrahedron 2005, 61, 2521.
doi: 10.1016/j.tet.2004.12.063 |
|
| [19] |
Marshall J. A. Chem. Rev. 1996, 96, 31.
pmid: 11848743 |
| [20] |
Roy U. K.; Roy S. Chem. Rev. 2010, 110, 2472.
doi: 10.1021/cr800336a |
| [21] |
Shen Z.-L.; Wang S.-Y.; Chok Y.-K.; Xu Y.-H.; Loh T.-P. Chem. Rev. 2013, 113, 271.
doi: 10.1021/cr300051y |
| [22] |
Tan K.-T.; Chng S.-S.; Cheng H.-S.; Loh T.-P. J. Am. Chem. Soc. 2003, 125, 2958.
doi: 10.1021/ja029276s |
| [23] |
Zhang J.; Blazecka P. G.; Berven H.; Belmont D. Tetrahedron Lett. 2003, 44, 5579.
doi: 10.1016/S0040-4039(03)01380-7 |
| [24] |
Wang Z.; Zha Z.; Zhou C. Org. Lett. 2002, 4, 1683.
doi: 10.1021/ol0257326 |
| [25] |
Zha Z.; Xie Z.; Zhou C.; Chang M.; Wang Z. New J. Chem. 2003, 27, 1297.
doi: 10.1039/b303187j |
| [26] |
(a) Ravindra B.; Das B. G.; Ghorai P. Org. Lett. 2014, 16, 5580.
doi: 10.1021/ol502614n pmid: 25337660 |
|
(b) Kumar P.; Shirke R. P.; Yadav S.; Ramasastry S. S. V. Org. Lett. 2021, 23, 4909.
doi: 10.1021/acs.orglett.1c01671 pmid: 25337660 |
|
| [27] |
Tripathi C. B.; Mukherjee S. Angew. Chem., Int. Ed. 2013, 52, 8450.
doi: 10.1002/anie.v52.32 |
| [1] | Feng Wang, Danfeng Huang, Pengfei Zhao, Ming Yang, Tongyu Han, Kehu Wang, Junjiao Wang, Yingpeng Su, Yulai Hu. Study on the Allylation of Benzol[e][1,2,3]oxathiazine-2,2-dioxides [J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 507-518. |
| [2] | Zhao Zhuanxia, Wang Junjiao, Huang Danfeng, Yang Zheng, Zhao Fangxia, Hu Yongqin, Xu Weigang, Hu Yulai. Study on Tin Powder-Promoted Allylation of 3-Aryl-3-hydroxy-2-oxindoles [J]. Chinese Journal of Organic Chemistry, 2020, 40(7): 2026-2034. |
| [3] | Liu Jiaxin, Huang Danfeng, Wang Xiaoping, Zong Wuzhong, Su Yingpeng, Wang Kehu, Hu Yulai. Tin Powder Promoted Synthesis of α-Trifluoromethyl Homoallylic Hydrazides [J]. Chin. J. Org. Chem., 2019, 39(6): 1767-1775. |
| [4] | Yang Zheng, Huang Danfeng, Wen Lan, Wang Juanjuan, Wang Kehu, Hu Yulai. Tin Powder-Promoted “One-Pot” Synthesis of α-Methylene-γ-butyrolactones [J]. Chin. J. Org. Chem., 2018, 38(7): 1725-1732. |
| [5] | Wang Kehu, Wang Yalin, Yin Xuejiao, Peng Xiansha, Huang Danfeng, Su Yingpeng, Hu Yulai. Tin-Promoted One-Pot Synthesis of Aryl/Trifluoromethyl Group Substituted Homoallylic N-Acylhydrazines [J]. Chin. J. Org. Chem., 2017, 37(7): 1764-1773. |
| [6] | Chang Qing, Kang Juan, Zhang Weigang, Wang Juanjuan, Huang Danfeng, Wang Kehu, Su Yingpeng, Hu Yulai. Tin-Mediated Preparation of Allylic α-Acylhydrazino Esters [J]. Chin. J. Org. Chem., 2016, 36(12): 2920-2927. |
| [7] | Xia Xiaowen, Ji Shilong, Wang Fengjiao, Huang Danfeng, Wang Ke-Hu, Su Yingpeng, Hu Yulai. Synthesis of Ethyl α-Methylene-γ-amino Carboxylates Promoted by Tin Powder [J]. Chin. J. Org. Chem., 2015, 35(5): 1040-1045. |
| [8] | Lu Ailing, Wang Fengjiao, Huang Danfeng, Wang Kehu, Su Yingpeng, X? Yanli, Hu Yulai . Tin-Mediated “One-Pot” Synthesis of Homoallylhydrazides from Aldehydes, Aryl Acylhydrazines and Allyl Bromide [J]. Chin. J. Org. Chem., 2014, 34(5): 948-955. |
| [9] | LI Jin-Heng*, LIAO Hua, LIANG Yun, YIN Du-Lin. Carbon Dioxide Promoted Allylation of Aldehydes and Ketones [J]. Chin. J. Org. Chem., 2005, 25(12): 1591-1593. |
| [10] | WANG Hui, PEI Wei-Wei*, WANG Yi. Synthesis of Novel Chiral Diallylic Diamine [J]. Chin. J. Org. Chem., 2005, 25(12): 1615-1618. |
| [11] | Zheng Yunfa;Lu Genliang;Zhang Yongmin. Sm/BiCl~3 system promoted synthesis of homoallylic amines in aqueous media [J]. Chin. J. Org. Chem., 2000, 20(4): 551-552. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||