Chinese Journal of Organic Chemistry ›› 2024, Vol. 44 ›› Issue (4): 1151-1159.DOI: 10.6023/cjoc202310017 Previous Articles Next Articles
REVIEWS
谌泽亚, 李诗瑶, 杨留攀*( ), 王力立*(
), 王力立*( ), 姚欢*(
), 姚欢*( )
)
收稿日期:2023-10-20
修回日期:2023-11-15
发布日期:2023-11-30
基金资助:
Zeya Shen, Shiyao Li, Liupan Yang( ), Lili Wang(
), Lili Wang( ), Huan Yao(
), Huan Yao( )
)
Received:2023-10-20
Revised:2023-11-15
Published:2023-11-30
Contact:
E-mail: Supported by:Share
Zeya Shen, Shiyao Li, Liupan Yang, Lili Wang, Huan Yao. Research Progress on the Construction and Application of Macrocyclic Fluorescent Sensing Platform Based on Indicator Displacement Assay[J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1151-1159.
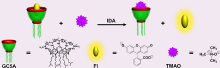
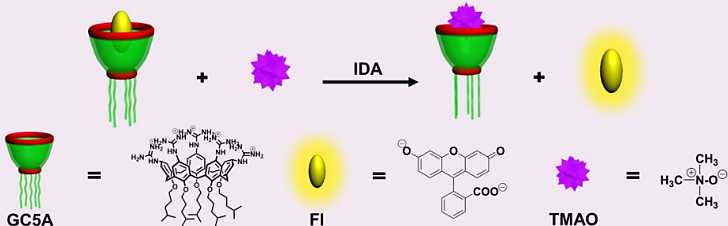
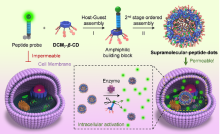
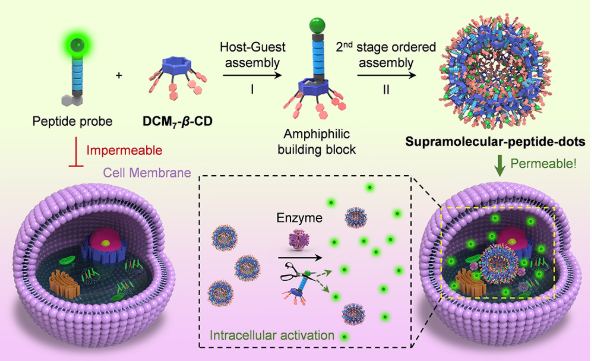
| [1] |
Guo C.; Sedgwick A. C.; Hirao T.; Sessler J. L. Coord. Chem. Rev. 2021, 427, 213560.
doi: 10.1016/j.ccr.2020.213560 |
| [2] |
Wu D.; Sedgwick A. C.; Gunnlaugsson T.; Akkaya E. U.; Yoon J.; James T. D. Chem. Soc. Rev. 2017, 46, 7105.
doi: 10.1039/C7CS00240H |
| [3] |
Mako T. L.; Racicot J. M.; Levine M. Chem. Rev. 2018, 119, 322.
doi: 10.1021/acs.chemrev.8b00260 |
| [4] |
Brzechwa-Chodzyńska A.; Drożdż W.; Harrowfield J.; Stefankiewicz A. R. Coord. Chem. Rev. 2021, 434, 213820.
doi: 10.1016/j.ccr.2021.213820 |
| [5] |
He Y.; Chen L.; He R.; Zhong K.; Tang L. Chin. J. Org. Chem. 2022, 42, 785. (in Chinese)
doi: 10.6023/cjoc202108024 |
|
(何雨晴, 陈琳, 贺瑞丽, 钟克利, 汤立军, 有机化学, 2022, 42, 785.)
doi: 10.6023/cjoc202108024 |
|
| [6] |
Tian X.; Zuo M.; Niu P.; Wang K.; Hu X. Chin. J. Org. Chem., 2020, 40, 1823. (in Chinese)
doi: 10.6023/cjoc202003066 |
|
(田雪琪, 左旻瓒, 牛蓬勃, 王开亚, 胡晓玉, 有机化学, 2020, 40, 1823.)
doi: 10.6023/cjoc202003066 |
|
| [7] |
Liu L.; Hao T.; Wu W.; Yang C. Chin. J. Org. Chem. 2023, 43, 2189. (in Chinese)
doi: 10.6023/cjoc202210020 |
|
(刘铃, 浩涛涛, 伍晚花, 杨成, 有机化学, 2023, 43, 2189.)
|
|
| [8] |
Cao X.; Gao A.; Hou J.; Yi T. Coord. Chem. Rev. 2021, 434, 213792.
doi: 10.1016/j.ccr.2021.213792 |
| [9] |
Rather I. A.; Ali R. Org. Biomol. Chem. 2021, 19, 5926.
doi: 10.1039/D1OB00518A |
| [10] |
Sedgwick A. C.; Brewster J. T.; Wu T.; Feng X.; Bull S. D.; Qian X.; Sessler J. L.; James T. D.; Anslyn E. V.; Sun X. Chem. Soc. Rev. 2021, 50, 9.
doi: 10.1039/c9cs00538b pmid: 33169731 |
| [11] |
Inouye M.; Hashimoto K.-I.; Isagawa K. J. Am. Chem. Soc. 1994, 116, 5517.
doi: 10.1021/ja00091a085 |
| [12] |
Pilicer S. L.; Bakhshi P. R.; Bentley K. W.; Wolf C. J. Am. Chem. Soc. 2017, 139, 1758.
doi: 10.1021/jacs.6b12056 |
| [13] |
Beatty M. A.; Borges-González J.; Sinclair N. J.; Pye A. T.; Hof F. J. Am. Chem. Soc. 2018, 140, 3500.
doi: 10.1021/jacs.7b13298 |
| [14] |
Minami T.; Liu Y.; Akdeniz A.; Koutnik P.; Esipenko N. A.; Nishiyabu R.; Kubo Y.; Anzenbacher P. J. Am. Chem. Soc. 2014, 136, 11396.
doi: 10.1021/ja504535q |
| [15] |
Minaker S. A.; Daze K. D.; Ma M. C. F.; Hof F. J. Am. Chem. Soc. 2012, 134, 11674.
doi: 10.1021/ja303465x pmid: 22703116 |
| [16] |
Liu Y.; Perez L.; Mettry M.; Easley C. J.; Hooley R. J.; Zhong W. J. Am. Chem. Soc. 2016, 138, 10746.
doi: 10.1021/jacs.6b05897 |
| [17] |
Wiskur S.-L. H., H.; Lavigne, J.-J.; Anslyn, E.-V. Acc. Chem. Res. 2001, 34, 963.
doi: 10.1021/ar9600796 |
| [18] |
Ghale G.; Nau W. M. Acc. Chem. Res. 2014, 47, 2150.
doi: 10.1021/ar500116d |
| [19] |
Dsouza R. N.; Pischel U.; Nau W. M. Chem. Rev. 2011, 111, 7941.
doi: 10.1021/cr200213s pmid: 21981343 |
| [20] |
Norouzy A.; Azizi Z.; Nau W. M. Angew. Chem., Int. Ed. 2015, 54, 792.
doi: 10.1002/anie.201407808 pmid: 25430503 |
| [21] |
Zheng Z.; Geng W.-C.; Gao J.; Wang Y.-Y.; Sun H.; Guo D.-S. Chem. Sci. 2018, 9, 2087.
doi: 10.1039/c7sc04989g pmid: 29675249 |
| [22] |
Yu H.; Geng W.-C.; Zheng Z.; Gao J.; Guo D.-S.; Wang Y. Theranostics 2019, 9, 4624.
doi: 10.7150/thno.33459 |
| [23] |
Zhang Y.; Yu H.; Chai S.; Chai X.; Wang L.; Geng W. C.; Li J. J.; Yue Y. X.; Guo D. S.; Wang Y. Adv. Sci. 2022, 9, 2104463.
doi: 10.1002/advs.v9.18 |
| [24] |
Yu H.; Chai X.; Geng W.-C.; Zhang L.; Ding F.; Guo D.-S.; Wang Y. Biosens. Bioelectron. 2021, 192, 113488.
doi: 10.1016/j.bios.2021.113488 |
| [25] |
Geng W. C.; Ye Z.; Zheng Z.; Gao J.; Li J. J.; Shah M. R.; Xiao L.; Guo D. S. Angew. Chem., Int. Ed. 2021, 60, 19614.
doi: 10.1002/anie.v60.36 |
| [26] |
Tian J.-H.; Hu X.-Y.; Hu Z.-Y.; Tian H.-W.; Li J.-J.; Pan Y.-C.; Li H.-B.; Guo D.-S. Nat. Commun. 2022, 13, 4293.
doi: 10.1038/s41467-022-31986-x |
| [27] |
Gutsche C. D.; Muthukrishnan R. J. Org. Chem. 1978, 43, 4905.
doi: 10.1021/jo00419a052 |
| [28] |
Bakirci H.; Nau W. M. Adv. Funct. Mater. 2006, 16, 237.
doi: 10.1002/adfm.v16:2 |
| [29] |
Yang L.; Xie X.; Cai L.; Ran X.; Li Y.; Yin T.; Zhao H.; Li C.-P. Biosens. Bioelectron. 2016, 82, 146.
doi: 10.1016/j.bios.2016.04.005 |
| [30] |
Geng W. C.; Jia S.; Zheng Z.; Li Z.; Ding D.; Guo D. S. Angew. Chem., Int. Ed. 2019, 58, 2377.
doi: 10.1002/anie.v58.8 |
| [31] |
Duan Q.; Chen R.; Deng S.; Yang C.; Ji X.; Qi G.; Li H.; Li X.; Chen S.; Lou M.; Lu K. Front. Chem. 2023, 11, 1124705.
doi: 10.3389/fchem.2023.1124705 |
| [32] |
Liu S. R., C.; Mukhopadhyay, P.; Chakrabarti, S.; Zavalij, P. Y.; Isaacs, L. J. Am. Chem. Soc. 2005, 217, 15959.
|
| [33] |
Liu M.; Zhou Y.; Chen L.; Bian B.; Xiao X.; Tao Z. Chin. Chem. Lett. 2021, 32, 375.
doi: 10.1016/j.cclet.2020.03.042 |
| [34] |
Lin R.-L.; Liu J.-X.; Chen K.; Redshaw C. Inorg. Chem. Front. 2020, 7, 3217.
doi: 10.1039/D0QI00529K |
| [35] |
Hennig A.; Bakirci H.; Nau W. M. Nat. Methods 2007, 4, 629.
doi: 10.1038/nmeth1064 |
| [36] |
Nau W. M. G., G.; Hennig, A.; Bakirci, H.; Bailey, D. M. J. Am. Chem. Soc. 2009, 131, 11558.
doi: 10.1021/ja904165c |
| [37] |
Yin T.; Zhang S.; Li M.; Redshaw C.; Ni X.-L. Sens. Actuators, B 2019, 281, 568.
doi: 10.1016/j.snb.2018.10.136 |
| [38] |
Sinn S.; Spuling E.; Bräse S.; Biedermann F. Chem. Sci. 2019, 10, 6584.
doi: 10.1039/C9SC00705A |
| [39] |
Wang C.; Tang Q.; Xi Y.; Yang M.; Li T.; Huang Y.; Tao Z. Chin. J. Org. Chem., 2018, 38, 1394. (in Chinese)
doi: 10.6023/cjoc201711018 |
|
(王成会, 唐青, 席芸芸, 杨梅, 李涛, 黄英, 陶朱, 有机化学, 2018, 38, 1394.)
doi: 10.6023/cjoc201711018 |
|
| [40] |
Barba-Bon A.; Pan Y.-C.; Biedermann F.; Guo D.-S.; Nau W. M.; Hennig A. J. Am. Chem. Soc. 2019, 141, 20137.
doi: 10.1021/jacs.9b09563 pmid: 31739668 |
| [41] |
Hu C.; Jochmann T.; Chakraborty P.; Neumaier M.; Levkin P. A.; Kappes M. M.; Biedermann F. J. Am. Chem. Soc. 2022, 144, 13084.
doi: 10.1021/jacs.2c01520 |
| [42] |
Ogoshi T. K., S.; Fujinami, S.; Yamagishi, T.; Nakamoto, Y. J. Am. Chem. Soc. 2008, 130, 5022.
doi: 10.1021/ja711260m |
| [43] |
Xu X.; Jerca V. V.; Hoogenboom R. Angew. Chem., Int. Ed. 2020, 59, 6314.
doi: 10.1002/anie.v59.16 |
| [44] |
Murray J.; Kim K.; Ogoshi T.; Yao W.; Gibb B. C. Chem. Soc. Rev. 2017, 46, 2479.
doi: 10.1039/c7cs00095b pmid: 28338130 |
| [45] |
Ogoshi T.; Yamagishi T.-a.; Nakamoto Y. Chem. Rev. 2016, 116, 7937.
doi: 10.1021/acs.chemrev.5b00765 |
| [46] |
Strutt N. L.; Zhang H.; Schneebeli S. T.; Stoddart J. F. Acc. Chem. Res. 2014, 47, 2631.
doi: 10.1021/ar500177d |
| [47] |
Bojtár M.; Kozma J.; Szakács Z.; Hessz D.; Kubinyi M.; Bitter I. Sens. Actuators, B 2017, 248, 305.
doi: 10.1016/j.snb.2017.03.163 |
| [48] |
Hua B.; Shao L.; Zhang Z.; Sun J.; Yang J. Sens. Actuators, B. 2018, 255, 1430.
doi: 10.1016/j.snb.2017.08.141 |
| [49] |
Paudics A.; Hessz D.; Bojtár M.; Bitter I.; Horváth V.; Kállay M.; Kubinyi M. Sens. Actuators, B 2022, 369, 132364.
doi: 10.1016/j.snb.2022.132364 |
| [50] |
Yang L.; Zhao H.; Li Y.; Zhang Y.; Ye H.; Zhao G.; Ran X.; Liu F.; Li C.-P. Biosens. Bioelectron. 2017, 87, 737.
doi: 10.1016/j.bios.2016.09.044 |
| [51] |
Lee J. Y.; Root H. D.; Ali R.; An W.; Lynch V. M.; Bähring S.; Kim I. S.; Sessler J. L.; Park J. S. J. Am. Chem. Soc. 2020, 142, 19579.
doi: 10.1021/jacs.0c08106 |
| [52] |
Jiao J.-B.; Wang G.-Z.; Hu X.-L.; Zang Y.; Maisonneuve S.; Sedgwick A. C.; Sessler J. L.; Xie J.; Li J.; He X.-P.; Tian H. J. Am. Chem. Soc. 2019, 142, 1925.
doi: 10.1021/jacs.9b11207 |
| [53] |
Sierra A. F.; Hernández-Alonso D.; Romero M. A.; González-Delgado J. A.; Pischel U.; Ballester P. J. Am. Chem. Soc. 2020, 142, 4276.
doi: 10.1021/jacs.9b12071 |
| [54] |
Yao H.; Li S.-Y.; Zhang H.; Pang X.-Y.; Lu J.-L.; Chen C.; Jiang W.; Yang L.-P.; Wang L.-L. Chem. Commun. 2023, 59, 5411.
doi: 10.1039/D2CC06622J |
| [55] |
Yao H.; Qin J.; Wang Y.-F.; Wang S.-M.; Yi L.-H.; Li S.-Y.; Du F.; Yang L.-P.; Wang L.-L. Chin. Chem. Lett. 2023, doi: 10.1016/j.cclet.2023.109154.
|
| [56] |
Hu C.; Grimm L.; Prabodh A.; Baksi A.; Siennicka A.; Levkin P. A.; Kappes M. M.; Biedermann F. Chem. Sci. 2020, 11, 11142.
doi: 10.1039/D0SC03079A |
| [57] |
Bockus A.-T. S., L.; Grice, A.-G.; Ali, O.-A.; Young, C.-C.; Mobley, W.; Leek, A.; Roberts, J.-L.; Vinciguerra, B.; Isaacs, L.; Urbach, A.-R. J. Am. Chem. Soc. 2016, 138, 16549.
doi: 10.1021/jacs.6b11140 |
| [58] |
Das Saha N.; Pradhan S.; Sasmal R.; Sarkar A.; Berač C. M.; Kölsch J. C.; Pahwa M.; Show S.; Rozenholc Y.; Topçu Z.; Alessandrini V.; Guibourdenche J.; Tsatsaris V.; Gagey-Eilstein N.; Agasti S. S. J. Am. Chem. Soc. 2022, 144, 14363.
doi: 10.1021/jacs.2c05969 |
| [1] | Xiao Zhang, Mixia Hu, Yanqing Du, Fengying Liang, Xiaoying Zhang, Chaolu Eerdun. Research Progress on Anion-π Interactions [J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1181-1196. |
| [2] | Fen Li, Chuanzhi Liu, Zhiyuan Hu, Panpan Luo, Rongzheng Cui, Yanke Huang, Xinming Liu, Lantao Liu, Wei Wu. Intermolecular Halogen and Hydrogen Bonding-Controlled Self-Assembly of Network Structures [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 705-711. |
| [3] | Huiming Lu, Lamaocao Ma, Hengchang Ma. Research Progress and Prospect of Aggregation-Induced Emission Supramolecular Luminescence Materials [J]. Chinese Journal of Organic Chemistry, 2023, 43(12): 4075-4105. |
| [4] | Jingjing Guo, Minjie Guo. Progress in Design and Application of Supramolecular Fluorescent Systems Based on Difluoroboron-Dipyrromethene and Macrocyclic Compounds [J]. Chinese Journal of Organic Chemistry, 2021, 41(8): 2946-2963. |
| [5] | Tian Xueqi, Zuo Minzan, Niu Pengbo, Wang Kaiya, Hu Xiaoyu. Research Advances of Host-Guest Supramolecular Self-Assemblies with Aggregration-Induced Emission Effect and Their Applications in Biomedical Field [J]. Chinese Journal of Organic Chemistry, 2020, 40(7): 1823-1834. |
| [6] | Hu Zhixiong, Sun Dongdong, Han Xie, Liu Simin. Molecular Recognition of Cucurbit[10]uril toward Planar d8 and d10 Transition Metal Complexes [J]. Chinese Journal of Organic Chemistry, 2020, 40(5): 1361-1366. |
| [7] | Xiao Tangxin, Zhou Ling, Wei Xiaoyan, Li Zhengyi, Sun Xiaoqiang. Supramolecular Copolymers Driven by Quadruple Hydrogen Bonding and Host-Guest Interactions [J]. Chinese Journal of Organic Chemistry, 2020, 40(4): 944-949. |
| [8] | Li Jing, Han Ying, Chen Chuanfeng. Recent Advances in Novel Macrocyclic Arenes [J]. Chinese Journal of Organic Chemistry, 2020, 40(11): 3714-3737. |
| [9] | Qi Lijie, Ding Yihan, Xiao Tangxin, Wu Haoran, Diao Kai, Bao Cheng, Shen Yong, Li Zhengyi, Sun Xiaoqiang, Wang Leyong. Supramolecular Self-Assembly of Dioxyphenylene Bridged Ureidopyrimidinone Derivatives [J]. Chinese Journal of Organic Chemistry, 2020, 40(11): 3847-3852. |
| [10] | Li Zhenyi, Hou Nana, Shao Wei, Xiao Shoujun, Lin Chen, Wang Leyong. Host-Guest Interaction between Water-Soluble Pillar[6]arene and 1,1'-Disubstituted Ferrocene Derivatives [J]. Chin. J. Org. Chem., 2018, 38(8): 2002-2007. |
| [11] | Xiao Liwei, Ren Ping, Jing Xuemin, Ren Lilei, Li Zheng, Dai Fucai. Application in Molecular Recognition of 1,2,3-Trizole Derivatives [J]. Chin. J. Org. Chem., 2017, 37(12): 3085-3095. |
| [12] | Peng Long, Liu Huijun, Hu Cheng, Sun Yunkai, Long Wei. Terephthaloyl Chloride Bridged Bis(β-cyclodextrin) and Their Synergetic Bonding Behaviors with Dyes [J]. Chin. J. Org. Chem., 2015, 35(6): 1330-1334. |
| [13] | Fan Wenjia, Chen Lijun, Yang Haibo. Supramolecular Organometallic Gels Based on Neutral Platinum-Acetylide Moiety [J]. Chin. J. Org. Chem., 2015, 35(3): 578-587. |
| [14] | Weng Guan-Huan, Zhu Bin, Ye Yang, Li Shijun. Acid/Base-Controllable Molecular Machines and Molecular Switches [J]. Chin. J. Org. Chem., 2015, 35(2): 309-324. |
| [15] | Yao Jiabin, Xu Mengyu, Yan Zhiqiang, Wu Wanhua, Yang Cheng. Supramolecular Photochirogenesis with Cyclodextrins [J]. Chin. J. Org. Chem., 2014, 34(1): 26-35. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||