Chinese Journal of Organic Chemistry ›› 2024, Vol. 44 ›› Issue (4): 1124-1150.DOI: 10.6023/cjoc202309012 Previous Articles Next Articles
REVIEWS
郭凯杰a, 符昕姝a, 李靖a, 陈艳a, 胡美丽a, 堵锡华a, 谢屿阳b,*( ), 何燕a,*(
), 何燕a,*( )
)
收稿日期:2023-09-11
修回日期:2023-12-06
发布日期:2023-12-15
基金资助:
Kaijie Guoa, Xinshu Fua, Jing Lia, Yan Chena, Meili Hua, Xihua Dua, Yuyang Xieb( ), Yan Hea(
), Yan Hea( )
)
Received:2023-09-11
Revised:2023-12-06
Published:2023-12-15
Contact:
E-mail: Supported by:Share
Kaijie Guo, Xinshu Fu, Jing Li, Yan Chen, Meili Hu, Xihua Du, Yuyang Xie, Yan He. Recent Advances in Transition-Metal-Catalyzed C—S Bond Activation and Transformations[J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1124-1150.
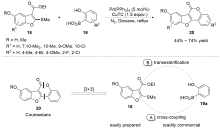
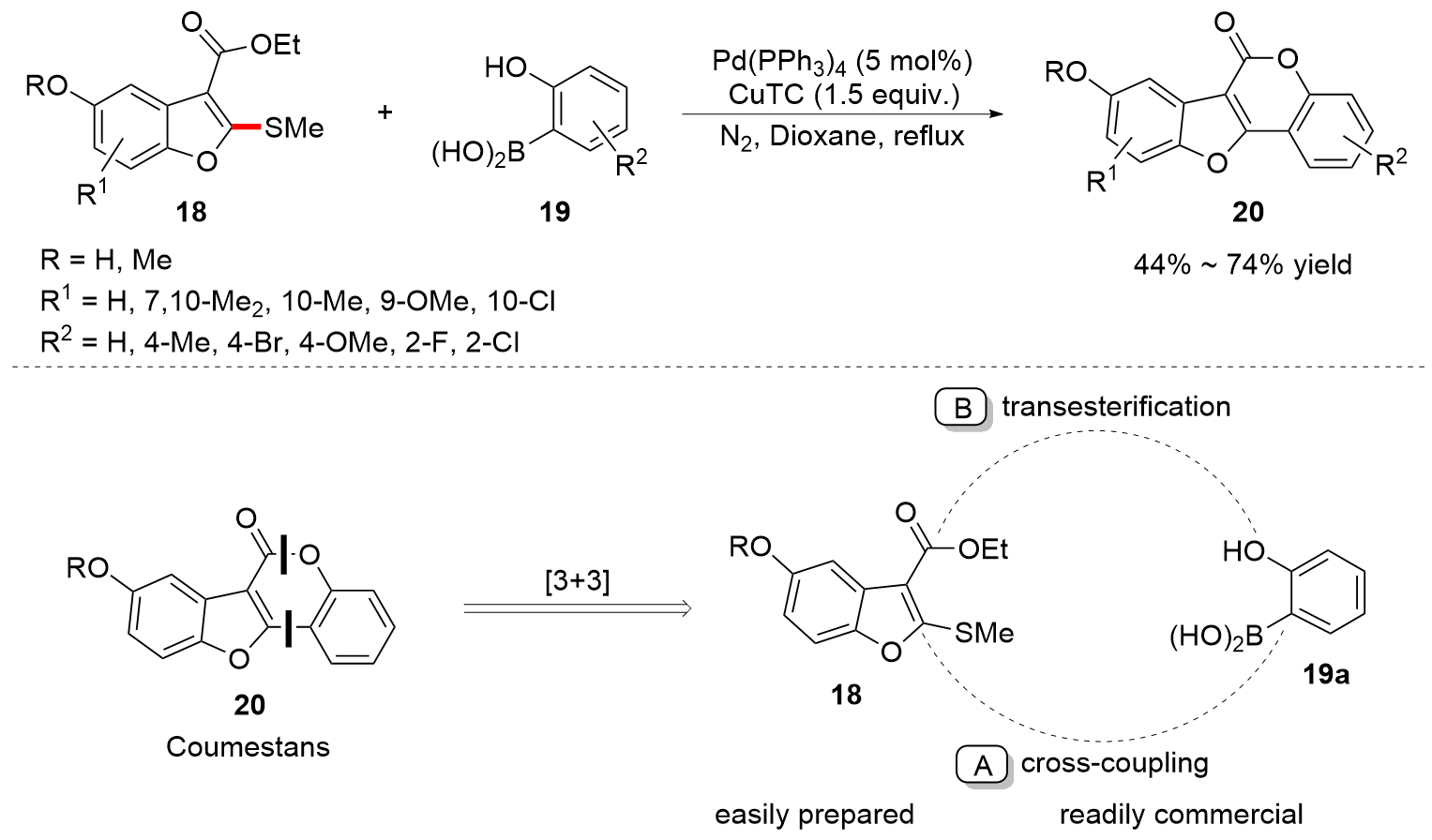
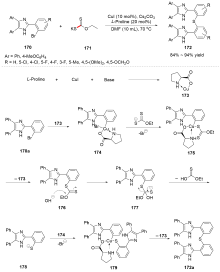

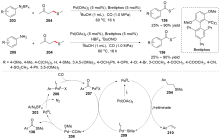
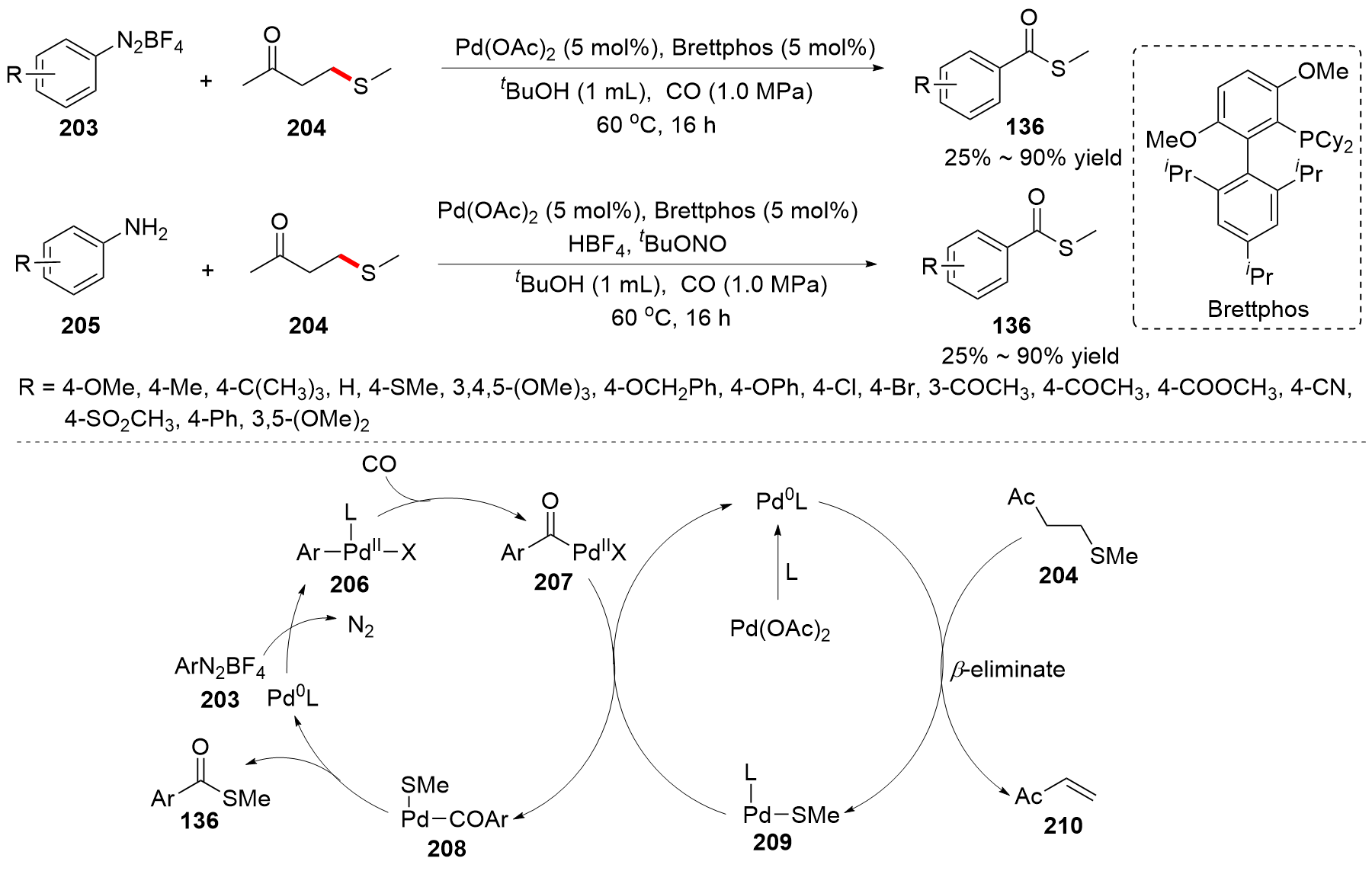

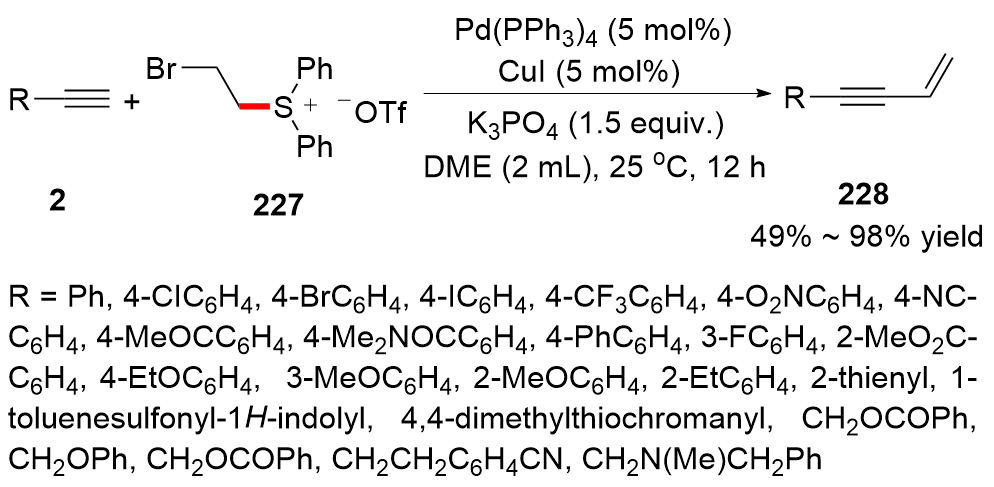
| [1] |
(a) Wächtershäuser G. Trans. R. Soc. B 2006, 361, 1787.
doi: 10.1098/rstb.2006.1904 pmid: 20704269 |
|
(b) Harpp D. N.; Vines S. M.; Montillier J. P.; Chan T. H. J. Org. Chem. 1976, 41, 3987.
doi: 10.1021/jo00887a012 pmid: 20704269 |
|
|
(c) Grochowski M. R.; Li T.; Brennessel W. W.; Jones W. D. J. Am. Chem. Soc. 2010, 132, 12412.
doi: 10.1021/ja104158h pmid: 20704269 |
|
| [2] |
(a) Boyd D. A. Angew. Chem., Int. Ed. 2016, 55, 15486.
doi: 10.1002/anie.v55.50 |
|
(b) Liu J.; Yang J.; Yang Q.; Wang G.; Li Y. Adv. Funct. Mater. 2005, 15, 1297.
doi: 10.1002/(ISSN)1616-3028 |
|
|
(c) Dondoni A. Angew. Chem., Int. Ed. 2008, 120, 9133.
doi: 10.1002/ange.v120:47 |
|
| [3] |
(a) Natarajan A.; Guo Y.; Harbinski F.; Fan Y.-H.; Chen H.; Luus L.; Diercks J.; Aktas H.; Chorev M.; Halperin J. A. J. Med. Chem. 2004, 47, 4979.
pmid: 19588965 |
|
(b) Cole D. C.; Lennox W. J.; Lombardi S.; Ellingboe J. W.; Bernotas R. C.; Tawa G. J.; Mazandarani H.; Smith D. L.; Zhang G.; Coupet J.; Schechter L. E. J. Med. Chem. 2005, 48, 353.
doi: 10.1021/jm049243i pmid: 19588965 |
|
|
(c) Banerjee M.; Poddar A.; Mitra G.; Surolia A.; Owa T.; Bhattacharyya B. J. Med. Chem. 2005, 48, 547.
pmid: 19588965 |
|
|
(d) Feng E.; Huang H.; Zhou Y.; Ye D.; Jiang H.; Liu H. J. Comb. Chem. 2010, 12, 422.
doi: 10.1021/cc9001839 pmid: 19588965 |
|
|
(e) Gao G.-Y.; Colvin A. J.; Chen Y.; Zhang X. P. J. Org. Chem. 2004, 69, 8886.
doi: 10.1021/jo048552d pmid: 19588965 |
|
|
(f) Inamoto K.; Hasegawa C.; Hiroya K.; Doi T. Org. Lett. 2008, 10, 5147.
doi: 10.1021/ol802033p pmid: 19588965 |
|
|
(g) Jegelka M.; Plietker B. Org. Lett. 2009, 11, 3462.
doi: 10.1021/ol901297s pmid: 19588965 |
|
|
(h) Niu P.; Kang J.; Tian X.; Song L.; Liu H.; Wu J.; Yu W.; Chang J. J. Org. Chem. 2015, 80, 1018.
doi: 10.1021/jo502518c pmid: 19588965 |
|
| [4] |
(a) Fang R.; Xu J.; Wang D.-W. Energy Environ. Sci. 2020, 13, 432.
doi: 10.1039/C9EE03408K |
|
(b) Shi F.; Yu J.; Chen C.; Lau S. P.; Lv W.; Xu Z.-L. J. Mater. Chem. A 2022, 10, 19412.
doi: 10.1039/D2TA02217F |
|
|
(c) Li M.; Chen H.; Wang Y.; Chen X.; Wu J.; Su J.; Wang M.; Li X.; Li C.; Ma L.; Li X.; Chen Y. J. Mater. Chem. A 2023, 11, 11721.
doi: 10.1039/D3TA01803B |
|
| [5] |
(a) Luque R. Curr. Org. Synth. 2011, 8, 1.
doi: 10.2174/157017911794407665 |
|
(b) Ornellas S. D.; Storr T. E.; Williams T. J.; Baumann C. G.; Fairlamb I. J. S. Curr. Org. Synth. 2011, 8, 79.
doi: 10.2174/157017911794407656 |
|
|
(c) Wang L.; He W.; Yu Z. Chem. Soc. Rev. 2013, 42, 599.
doi: 10.1039/C2CS35323G |
|
|
(d) Modha S. G.; Mehta V. P.; Eycken E. V. Chem. Soc. Rev. 2013, 42, 5042.
doi: 10.1039/c3cs60041f |
|
|
(e) Lou J.; Wang Q.; Wu P.; Wang H.; Zhou Y. G.; Yu Z. Chem. Soc. Rev. 2020, 49, 4307.
doi: 10.1039/C9CS00837C |
|
|
(f) Huang S.; Wang M.; Jiang X. Chem. Soc. Rev. 2022, 51, 8351.
doi: 10.1039/D2CS00553K |
|
|
(g) Otsuka S.; Nogi K.; Yorimitsu H. Top. Curr. Chem. 2018, 376, 13.
|
|
| [6] |
(a) Li Y.; Wang H.; Wang Z.; Alhumade H.; Huang Z.; Lei A. Chem. Sci. 2023, 14, 372.
doi: 10.1039/D2SC05507D |
|
(b) Tyagi A.; Taneja N.; Khan J.; Hazra C. K. Adv. Synth. Catal. 2023, 365, 1247.
doi: 10.1002/adsc.v365.8 |
|
|
(c) Liang D.; Wang M.; Bekturhun B.; Xiong B.; Liu Q. Adv. Synth. Catal. 2010, 352, 1593.
doi: 10.1002/adsc.v352:10 |
|
|
(d) Liu Y.; Wang M.; Yuan H.; Liu Q. Adv. Synth. Catal. 2010, 352, 884.
doi: 10.1002/adsc.v352:5 |
|
|
(e) Liu Y.; Liu J.; Wang M.; Liu J.; Liu Q. Adv. Synth. Catal. 2012, 354, 2678.
doi: 10.1002/adsc.v354.14/15 |
|
|
(f) Yu H.; Yu Z. Angew. Chem. 2009, 121, 2973.
doi: 10.1002/ange.v121:16 |
|
|
(g) Dong Y.; Wang M.; Liu J.; Ma W.; Liu Q. Chem. Commun. 2011, 47, 7380.
doi: 10.1039/c1cc11382h |
|
|
(h) Verma R. K.; Verma G. K.; Shukla G.; Singh M. S. RSC Adv. 2012, 2, 2413.
doi: 10.1039/c2ra00987k |
|
|
(i) Jin W.; Du W.; Yang Q.; Yu H.; Chen J.; Yu Z. Org. Lett. 2011, 13, 4272.
doi: 10.1021/ol201620g |
|
| [7] |
Yang K.; Li Q.; Li Z.; Sun X. Chem. Commun. 2023, 59, 5343.
doi: 10.1039/D3CC00377A |
| [8] |
Liebeskind L. S.; Srogl J.; Savarin C.; Polanco C. Pure Appl. Chem. 2002, 74, 115.
doi: 10.1351/pac200274010115 |
| [9] |
Iwasaki M.; Fujino D.; Wada T.; Kondoh A.; Yorimitsu H.; Oshima K. Chem. Asian J. 2011, 6, 3190.
doi: 10.1002/asia.v6.12 |
| [10] |
Arisawa M.; Igarashi Y.; Tagami Y.; Yamaguchi M.; Kabuto C. Tetrahedron Lett. 2011, 52, 920
doi: 10.1016/j.tetlet.2010.12.065 |
| [11] |
Shibata T.; Mitake A.; Akiyamac Y.; Stephen K. K. Chem. Commun. 2017, 53, 9016.
doi: 10.1039/C7CC04997H |
| [12] |
Beletskaya I. P.; Ananikov V. P. Chem. Rev. 2022, 122, 16110.
doi: 10.1021/acs.chemrev.1c00836 pmid: 36112510 |
| [13] |
(a) Beletskaya I. P.; Alonso F.; Tyurin V. Coord Chem. Rev. 2019, 385, 137.
doi: 10.1016/j.ccr.2019.01.012 |
|
(b) Buchspies J.; Szostak M. Catalysts 2019, 9, 53.
doi: 10.3390/catal9010053 |
|
|
(c) Das P.; Linert W. Coord. Chem. Rev. 2016, 311, 1.
doi: 10.1016/j.ccr.2015.11.010 |
|
|
(d) Han F.-S. Chem. Soc. Rev. 2013, 42, 5270.
doi: 10.1039/c3cs35521g |
|
|
(e) Hooshmand S. E.; Heidari B.; Sedghi R.; Varma R. S. Green chem. 2019, 21, 381.
doi: 10.1039/c8gc02860e |
|
|
(f) Lennox A. J. J.; Lloyd-Jones G. C. Chem. Soc. Rev. 2014, 43, 412.
doi: 10.1039/C3CS60197H |
|
|
(g) Lamblin M.; Nassar-Hardy L.; Hierso J.-C.; Fouquet E.; Felpin F.-X. Adv. Synth. Catal. 2010, 352, 33.
doi: 10.1002/adsc.v352:1 |
|
| [14] |
Liu B.; Zheng G.; Liu X.; Xu C.; Liu J.; Wang M. Chem. Commun. 2013, 49, 2201.
doi: 10.1039/c3cc37571d |
| [15] |
Liu J.; Liu Y.; Du W.; Dong Y.; Liu J.; Wang M. J. Org. Chem. 2013, 78, 7293.
doi: 10.1021/jo400984h |
| [16] |
Dong Y.; Liu B.; Chen P.; Liu Q.; Wang M. Angew. Chem. 2014, 126, 3510.
doi: 10.1002/ange.v126.13 |
| [17] |
Otsuka S.; Fujino D.; Murakami K.; Yorimitsu H.; Osuka A. Chem. Eur. J. 2014, 20, 13146.
doi: 10.1002/chem.v20.41 |
| [18] |
Vasu D.; Yorimitsu H.; Osuka A. Synthesis 2015, 47, 3286.
doi: 10.1055/s-00000084 |
| [19] |
Liu B.; Chang J.; Zheng G.; Song X.; Wang M. Eur. J. Org. Chem. 2015, 4611.
|
| [20] |
Chang J.; Liu B.; Yang Y.; Wang M. Org. Lett. 2016, 18, 3984.
doi: 10.1021/acs.orglett.6b01780 pmid: 27498923 |
| [21] |
Wang S.-M.; Wang X.-Y.; Qin H.-L.; Zhang C.-P. Chem. Eur. J. 2016, 22, 6542.
doi: 10.1002/chem.v22.19 |
| [22] |
Iwasaki M.; Topolovčan N.; Hu H.; Nishimura Y.; Gagnot G.; Na nakorn R.; Yuvacharaskul R.; Nakajima K.; Nishihara Y. Org. Lett. 2016, 18, 1642.
doi: 10.1021/acs.orglett.6b00503 |
| [23] |
Wang Q.; Liu Z.; Lou J.; Yu Z. Org. Lett. 2018, 20, 6007.
doi: 10.1021/acs.orglett.8b02253 |
| [24] |
Otsuka S.; Nogi K.; Yorimitsu H. Angew. Chem., Int. Ed. 2018, 57, 6653.
doi: 10.1002/anie.v57.22 |
| [25] |
Minami H.; Nogi K.; Yorimitsu H. Org. Lett. 2019, 21, 2518.
doi: 10.1021/acs.orglett.9b00067 |
| [26] |
Uno D.; Nogi K.; Yorimitsu H. Org. Lett. 2019, 21, 8295.
doi: 10.1021/acs.orglett.9b03056 |
| [27] |
Xu J.-X.; Zhao F.; Wu X.-F. Org. Biomol. Chem. 2020, 18, 9796.
doi: 10.1039/D0OB02043E |
| [28] |
Delcaillau T.; Schmitt H. L.; Boehm P.; Falk E.; Morandi B. ACS Catal. 2022, 12, 6081.
doi: 10.1021/acscatal.2c01178 |
| [29] |
Yang S.; Yu X.; Poater A.; Cavallo L.; Cazin C. S. J.; Nolan S. P.; Szostak M. Org. Lett. 2022, 24, 9210.
doi: 10.1021/acs.orglett.2c03717 |
| [30] |
Mond J.; Langer C.; Quincke F. J. Chem. Soc., Trans. 1890, 57, 749.
doi: 10.1039/CT8905700749 |
| [31] |
Wilke G. Angew. Chem., Int. Ed. 1988, 27, 185.
|
| [32] |
Stephan Enthaler, C. I. S. M. W. Catal. Lett. 2013, 143, 424.
doi: 10.1007/s10562-013-0979-5 |
| [33] |
Yamamoto K.; Otsuka S.; Nogi K.; Yorimitsu H. ACS Catal. 2017, 7, 7623.
doi: 10.1021/acscatal.7b02347 |
| [34] |
Yamada K.; Yanagi T.; Yorimitsu H. Org. Lett. 2020, 22, 9712.
doi: 10.1021/acs.orglett.0c03782 pmid: 33300805 |
| [35] |
Inami T.; Kurahashi T.; Matsubara S. Synlett 2021, 32, 1948.
doi: 10.1055/s-0037-1610785 |
| [36] |
Shibata T.; Sekine A.; Akino M.; Ito M. Chem. Commun., 2021, 57, 9048.
doi: 10.1039/D1CC03226G |
| [37] |
Mai W.-P.; Sui H.-D.; Lv M.-X.; Lu K. J. Chem. Res. 2021, 45, 890.
doi: 10.1177/17475198211028114 |
| [38] |
Pan F.; Wang H.; Shen P.-X.; Zhao J.; Shi Z.-J. Chem. Sci. 2013, 4, 1573.
doi: 10.1039/c3sc22242j |
| [39] |
Uetake Y.; Niwa T.; Hosoya T. Org. Lett. 2016, 18, 2758.
doi: 10.1021/acs.orglett.6b01250 pmid: 27210907 |
| [40] |
(a) Sherry B. D.; Fürstner A. Acc. Chem. Res. 2008, 41, 1500.
doi: 10.1021/ar800039x |
|
(b) Czaplik W. M.; Mayer M.; Cvengros, Wangelin, J.; A. Jacobi von. ChemSusChem 2009, 2, 396.
doi: 10.1002/cssc.v2:5 |
|
|
(c) Piontek A.; Bisz E.; Szostak M. Angew. Chem., Int. Ed. 2018, 57, 11116.
doi: 10.1002/anie.v57.35 |
|
| [41] |
Blanksby S. J.; Ellison G. B. Acc. Chem. Res. 2003, 36, 255.
doi: 10.1021/ar020230d |
| [42] |
Lou J.; Wang Q.; Wu K.; Wu P.; Yu Z. Org. Lett. 2017, 19, 3287.
doi: 10.1021/acs.orglett.7b01431 |
| [43] |
Chen S.; Guo X.; Hou H.; Geng S.; Liu Z.; He Y.; Xue X.-S.; Feng Z. Angew. Chem., Int. Ed. 2023, 62, e202303470.
doi: 10.1002/anie.v62.25 |
| [44] |
Zhang Y.; Li T.-J.; Lv L.; Liu J.-Q.; Wang X.-S. J. Heterocyclic. Chem. 2022, 59, 67.
doi: 10.1002/jhet.v59.1 |
| [45] |
Tian Z.-Y.; Wang S.-M.; Jia S.-J.; Song H.-X.; Zhang C.-P. Org. Lett. 2017, 19, 5454.
doi: 10.1021/acs.orglett.7b02764 |
| [46] |
Li Y.; Wang H.; Wang Z.; Alhumade H.; Huang Z.; Lei A. Chem. Sci. 2023, 14, 372.
doi: 10.1039/D2SC05507D |
| [47] |
Nambo M.; Crudden C. M. Angew. Chem., Int. Ed. 2014, 53, 742.
doi: 10.1002/anie.v53.3 |
| [48] |
Simkó D. C.; Elekes P.; Pázmándi V.; Novák Z. Org. Lett. 2018, 20, 676.
doi: 10.1021/acs.orglett.7b03813 |
| [49] |
Li Y.; Bao G.; Wu X.-F. Chem. Sci. 2020, 11, 2187.
doi: 10.1039/C9SC05532K |
| [50] |
Tian Q.; Xu S.; Zhang C.; Liu X.; Wu X.; Li Y. J.Org. Chem. 2021, 86, 8797.
doi: 10.1021/acs.joc.1c00665 |
| [51] |
Yu H.; Zhao L.; Diao Q.; Li T.; Liao P.; Hou D.; Xin G. Synlett 2017, 28, 1828.
doi: 10.1055/s-0036-1588982 |
| [52] |
Zhang X.-S.; Zhang Y.-F.; Li Z.-W.; Luo F.-X.; Shi Z.-J. Angew. Chem., Int. Ed. 2015, 54, 5478.
doi: 10.1002/anie.v54.18 |
| [53] |
Ming X.-X.; Wu S.; Tian Z.-Y.; Song J.-W.; Zhang C.-P. Org. Lett. 2021, 23, 6795.
doi: 10.1021/acs.orglett.1c02379 |
| [1] | Chen-Long Li, Zhi-Xiang Yu. Progress in Transition-Metal-Catalyzed Carbonylative Cycloadditions Using Carbon Monoxide [J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1045-1068. |
| [2] | Hongqiong Zhao, Miao Yu, Dongxue Song, Qi Jia, Yingjie Liu, Yubin Ji, Ying Xu. Progress on Decarboxylation and Hydroxylation of Carboxylic Acids [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 70-84. |
| [3] | Xiaojing Hu, Feixiang Guo, Runqing Zhu, Bingqi Zhou, Tao Zhang, Lizhen Fang. Synthesis of p-Alkoxy Phenol and Its Application after Dearomatization [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2239-2244. |
| [4] | Guangli Xu, Jing Xu, Haidong Xu, Xiang Cui, Xingzhong Shu. Research Progress of Transition Metal Catalyzed Synthesis of 1,3- Conjugated Diene Compounds from Alkenes and Alkynes [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 1899-1933. |
| [5] | Kongchuan Wu, Kaihong Lu, Jianbin Lin, Huijun Zhang. Research Progress in Ortho-C—H Bond Functionalization of Rylene Diimides [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1000-1011. |
| [6] | Hairui Jia, Zaozao Qiu. Recent Advances in Transition Metal-Catalyzed B—H Bond Activation for Synthesis of o-Carborane Derivatives with B—Heteroatom Bond [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1045-1068. |
| [7] | Min Liu, Liping Qi, Dongbing Zhao. Recent Advances in Transition Metal-Catalyzed C—Si Bond Cleavage of Silacyclobutanes [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3508-3525. |
| [8] | Donghan Liu, Xihang Lu, Zhangmengjie Chai, Haoqi Yang, Yulin Sun, Fuchao Yu. Progress in Construction of 2H-Pyrrol-2-ones Skeleton [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 57-73. |
| [9] | Yuanhao Mao, Yanfeng Gao, Zhiwei Miao. Research Progress on the Asymmetric Cyclization Synthesis of Seven-Membered Rings via Transition Metal Catalysis [J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 1904-1924. |
| [10] | Luqi Liang, Lizhi Zhang, Yongli Peng, Hui Liu. Transition-Metal Catalyzed Coupling Reactions of gem-Dibromovinyl Derivatives [J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 1033-1060. |
| [11] | Chenguang Liu, Qiang Liu. Earth-Abundant Metal-Catalyzed Asymmetric Hydrogenation of Carbon-Nitrogen Unsaturated Bonds [J]. Chinese Journal of Organic Chemistry, 2022, 42(10): 3213-3220. |
| [12] | Haorui Wang, Mengchun Ye. Research Advance on Enantioselective Transition Metal-Catalyzed Hydroacylation Reactions [J]. Chinese Journal of Organic Chemistry, 2022, 42(10): 3152-3166. |
| [13] | Jian Zhang, Ying Chen, Quannan Wang, Jiahuan Shen, Yangzi Liu, Weiping Deng. Transition Metal-Catalyzed Asymmetric Cyclizations Involving Allyl or Propargyl Heteroatom-Dipole Precursors [J]. Chinese Journal of Organic Chemistry, 2022, 42(10): 3051-3101. |
| [14] | Xi Zhao, Yingcong Ou, Yan Liu, Keiji Maruoka, Qian Chen. Recent Progress in the Construction of S—S, P—S and C—S Bonds Involving O2-Initiated Sulfur-Centered Radicals [J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3366-3378. |
| [15] | Ci Li, Mingrui Li, Yuxing Xie, Yang Yu, Fei Huang. Progress in the Synthesis of Pyrrole-2-carboxylate Catalyzed by Transition Metals [J]. Chinese Journal of Organic Chemistry, 2021, 41(2): 594-610. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||