Chinese Journal of Organic Chemistry ›› 2024, Vol. 44 ›› Issue (6): 1862-1869.DOI: 10.6023/cjoc202401022 Previous Articles Next Articles
ARTICLES
李非凡a, 余康a, 倪传志b, 朱园园b,*( ), 曾婕c,*(
), 曾婕c,*( ), 古双喜a,c,*(
), 古双喜a,c,*( )
)
收稿日期:2024-01-16
修回日期:2024-05-08
发布日期:2024-03-28
基金资助:
Feifan Lia, Kang Yua, Chuanzhi Nib, Yuanyuan Zhub,*( ), Jie Zengc,*(
), Jie Zengc,*( ), Shuangxi Gua,c,*(
), Shuangxi Gua,c,*( )
)
Received:2024-01-16
Revised:2024-05-08
Published:2024-03-28
Contact:
* E-mail: Supported by:Share
Feifan Li, Kang Yu, Chuanzhi Ni, Yuanyuan Zhu, Jie Zeng, Shuangxi Gu. Chiral Fluorescent Probes for Determination of Both Concentration and Enantiomeric Composition of Amino Acids[J]. Chinese Journal of Organic Chemistry, 2024, 44(6): 1862-1869.
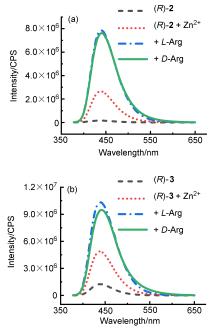

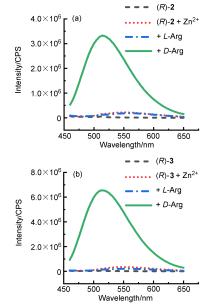

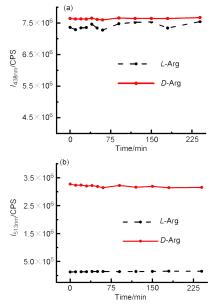




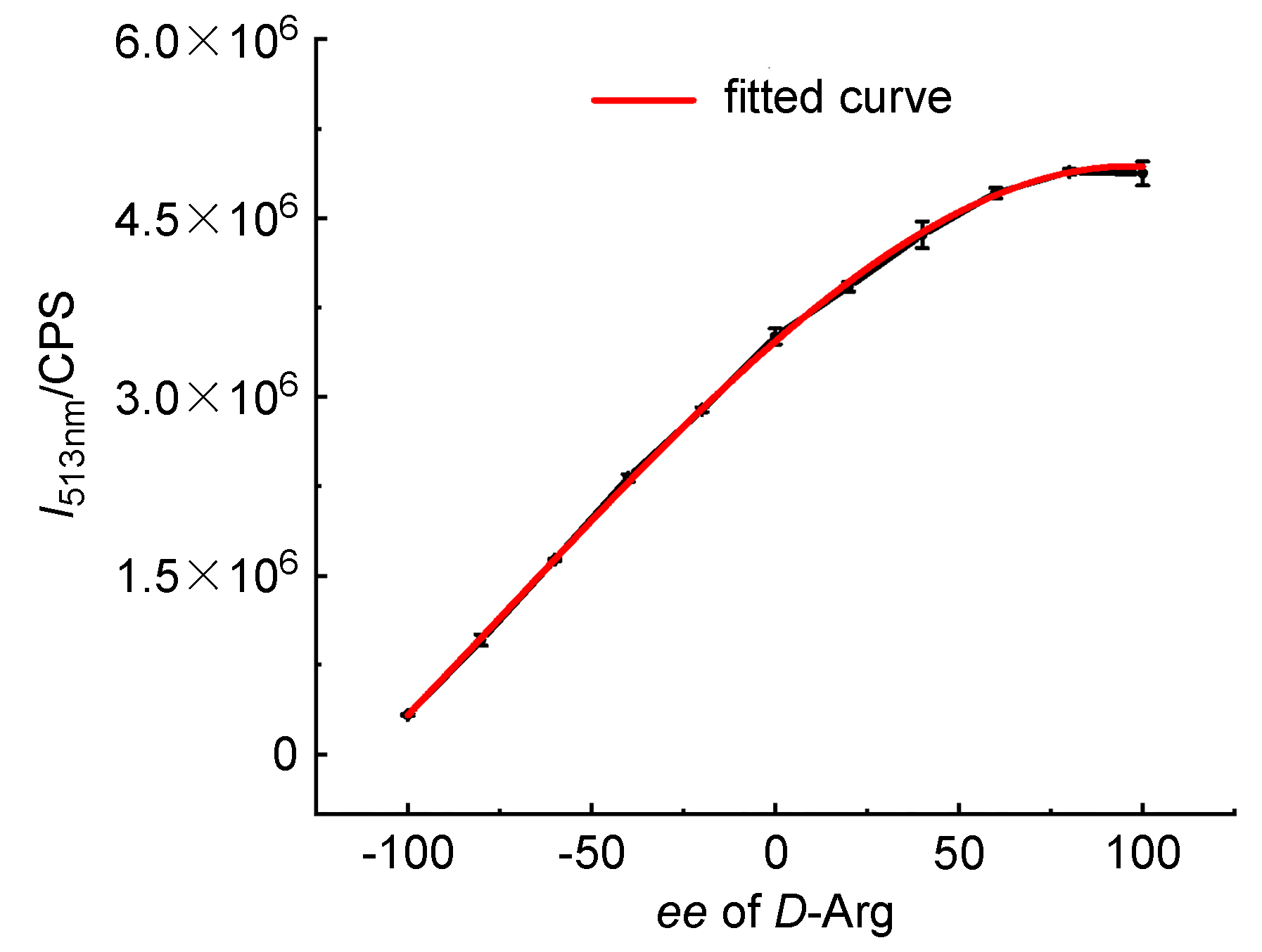
| Sample | ee of sample/% | Actual concentration/(mmol•L–1) | Determined concentrationa/(mmol•L–1) | Absolute error/(mmol•L–1) |
|---|---|---|---|---|
| 1 | 100 | 4 | 5.44 | 1.44 |
| 2 | –100 | 10 | 9.66 | 0.34 |
| 3 | –51 | 11 | 13.37 | 2.37 |
| 4 | –18 | 15 | 15.51 | 0.51 |
| 5 | 21 | 19 | 18.91 | 0.09 |
| 6 | 49 | 24 | 22.65 | 1.35 |
| 7 | –100 | 27 | 28.08 | 1.08 |
| 8 | 82 | 28 | 26.89 | 1.11 |
| Sample | ee of sample/% | Actual concentration/(mmol•L–1) | Determined concentrationa/(mmol•L–1) | Absolute error/(mmol•L–1) |
|---|---|---|---|---|
| 1 | 100 | 4 | 5.44 | 1.44 |
| 2 | –100 | 10 | 9.66 | 0.34 |
| 3 | –51 | 11 | 13.37 | 2.37 |
| 4 | –18 | 15 | 15.51 | 0.51 |
| 5 | 21 | 19 | 18.91 | 0.09 |
| 6 | 49 | 24 | 22.65 | 1.35 |
| 7 | –100 | 27 | 28.08 | 1.08 |
| 8 | 82 | 28 | 26.89 | 1.11 |
| Sample | Actual ee/% | Determined eea/% | Absolute error/% |
|---|---|---|---|
| 9 | –84 | –82.45 | 1.55 |
| 10 | –69 | –68.71 | 0.29 |
| 11 | –35 | –30.59 | 4.41 |
| 12 | 18 | 14.05 | 3.95 |
| 13 | 77 | 78.59 | 1.59 |
| Sample | Actual ee/% | Determined eea/% | Absolute error/% |
|---|---|---|---|
| 9 | –84 | –82.45 | 1.55 |
| 10 | –69 | –68.71 | 0.29 |
| 11 | –35 | –30.59 | 4.41 |
| 12 | 18 | 14.05 | 3.95 |
| 13 | 77 | 78.59 | 1.59 |
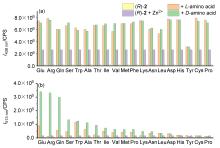

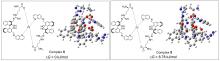

| [1] |
(a) Vellai, T. Nature 2021, 596, 192.
|
|
(b) Oh, S. F.; Praveena, T.; Song, H.; Yoo, J. S.; Jung, D. J.; Erturk-Hasdemi, D.; Hwang, Y. S.; Lee, C. C.; Le Nours, J.; Kim, H.; Lee, J.; Blumberg, R. S.; Rossjohn, J.; Park, S. B.; Kasper, D. L. Nature 2021, 600, 302.
|
|
|
(c) Gu, S.-X.; Wang, H.-F.; Zhu, Y.-Y.; Chen, F.-E. Pharm. Fronts 2020, 2, e79.
|
|
| [2] |
(a) Cheng, X.; Shen, C.; Dong, X.-Q.; Wang, C.-J. Chem. Commun. 2022, 58, 3142.
|
|
(b) Li, K.; Wang, L.; Zhen, S.; Zhu, L.; Yu, S.; Wu, Y.; Guo, H. Org. Chem. Front. 2023, 10, 5463.
|
|
|
(c) Nie, F.; Wang, K.-Z.; Yan, D. Nat. Commun. 2023, 14, 1654.
|
|
|
(d) Cai, J.; Liu, A.-A.; Shi, X.-H.; Fu, H.; Zhao, W.; Xu, L.; Kuang, H.; Xu, C.; Pang, D.-W. J. Am. Chem. Soc. 2023, 145, 24375.
|
|
| [3] |
Reviews on the determination of concentration and enantiomeric composition of amino acids by fluorescent probes: (a) Pu, L. Acc. Chem. Res. 2017, 50, 1032.
|
|
(b) Herrera, B. T.; Pilicer, S. L.; Anslyn, E. V.; Joyce, L. A.; Wolf, C. J. Am. Chem. Soc. 2018, 140, 10385.
|
|
|
(c) Yu, F.; Chen, Y.; Jiang, H.; Wang, X. Analyst 2020, 145, 6769.
|
|
| [4] |
Researches on the determination of concentration and enantiomeric composition of amino acids by fluorescent probes: (a) Wang, Q.; Wu, X.; Pu, L. Org. Lett. 2019, 21, 9036.
doi: 10.1021/acs.orglett.9b03437 pmid: 31663766 |
|
(b) Wen, K.; Yu, S.; Huang, Z.; Chen, L.; Xiao, M.; Yu, X.; Pu, L. J. Am. Chem. Soc. 2015, 137, 4517.
pmid: 31663766 |
|
|
(c) Iqbal, S.; Yu, S.; Jiang, L.; Wang, X.; Chen, Y.; Wang, Y.; Yu, X.; Pu, L. Chem.-Eur. J. 2019, 25, 9967.
pmid: 31663766 |
|
| [5] |
Determination of concentration and enantiomeric composition of chiral amines by other methods: (a) Bentley, K. W.; Wolf, C. J. Am. Chem. Soc. 2013, 135, 12200.
doi: 10.1021/ja406259p pmid: 23909867 |
|
(b) Nieto, S.; Lynch, V. M.; Anslyn, E. V.; Kim, H.; Chin, J. J. Am. Chem. Soc. 2008, 130, 9232.
pmid: 23909867 |
|
|
(c) Nieto, S.; Dragna, J. M.; Anslyn, E. V. Chem.-Eur. J. 2010, 16, 227.
pmid: 23909867 |
|
| [6] |
Non-BINOL-based chiral fluorescence probes: (a) Mei, X. F.; Wolf, C. Chem. Commun. 2004, 2078.
pmid: 17580908 |
|
(b) Upadhyay, S. P.; Pissurlenkar, R. R. S.; Coutinho, E. C.; Karnik, A. V. J. Org. Chem. 2007, 72, 5709.
pmid: 17580908 |
|
|
(c) Ghosn, M. W.; Wolf, C. J. Am. Chem. Soc. 2009, 131, 16360.
pmid: 17580908 |
|
| [7] |
Huang, Z.; Yu, S.; Wen, K.; Yu, X.; Pu, L. Chem. Sci. 2014, 5, 3457.
|
| [8] |
Zhu, Y.-Y.; Wu, X.-D.; Gu, S.-X.; Pu, L. J. Am. Chem. Soc. 2019, 141, 175.
|
| [9] |
Achiral fluorescent probes of Zn(II) with quinoline groups: (a) Hsieh, W. H.; Wan, C.-F.; Liao, D.-J.; Wu, A.-T. Tetrahedron Lett. 2012, 53, 5848.
|
|
(b) Kimura, E.; Koike, T. Chem. Soc. Rev. 1998, 27, 179.
|
|
|
(c) Zalewski, P. D.; Forbes, I. J.; Seamark, R. F.; Borlinghaus, R.; Betts, W. H.; Lincoln, S. F.; Ward, A. D. Chem. Biol. 1994, 1, 153.
|
|
|
(d) Zalewski, P. D.; Forbes, I. J.; Betts, W. H. Biochem. J. 1993, 296, 403.
|
|
|
(e) Huang, S.; Yang, B.; Tu, S. Comput. Theor. Chem. 2022, 1210, 113647.
|
|
|
(f) Gao, L.-L.; Li, S.-P.; Wang, Y.; Wu, W.-N.; Zhao, X.-L.; Li, H.-J.; Xu, Z.-H. Spectrochim. Acta, Part A 2020, 230, 118025.
|
| [1] | Lin Liu, Lin Chen, Xiaoling Hu, Keli Zhong, Jinglin Zhang, Lijun Tang. Application and Cell Imaging of Turn-On Fluorescent Probe for Hydrogen Sulfide Based on Benzopyran in Food Samples [J]. Chinese Journal of Organic Chemistry, 2024, 44(6): 2027-2032. |
| [2] | Wenyan Zhang, Dan Wang, Renjie Luo, Huiling Liu. Research Progress of Near-Infrared Fluorescent Surgical Navigation Probes [J]. Chinese Journal of Organic Chemistry, 2024, 44(6): 1760-1776. |
| [3] | Jidong Zhang, Yao Yang, Jie Zhang, Wei She. Detection of Zn(II) by Tetraphenylethyene Fluorescent Probe Based on Aggregation-Induced Emission (AIE)-Excited State Intramolecular Proton Transfer (ESIPT) Effect [J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1337-1342. |
| [4] | Xiaohong Cheng, Falong Liu, Jinbo Sun, Rui Zhang. An Ensemble-Based Fluorescent Probe for Real-Time and High Sensitive Detection of Hypochlorite [J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1284-1292. |
| [5] | Dongqing Xu, Haishan Tong, Jie Shen, Wanwei Qiu, Lisheng Qian. Construction of a Lipid Droplets Targeted Fluorescent Probe for Visualization of Liver Tumor Cells [J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1240-1246. |
| [6] | Xinyue Fang, Yawen Huang, Xinwei Hu, Zhixiong Ruan. Recent Progress in Electrochemical Modification of Amino Acids and Peptides [J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 903-926. |
| [7] | Chun Gao, Xin Liu, Minghui Wang, Shuxian Liu, Tingting Zhu, Yikang Zhang, Erjun Hao, Qiliang Yang. Advances in Asymmetric Electrochemical Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(3): 673-727. |
| [8] | Yingzhen Zhang, Dandan Jiang, Juanhua Li, Jingjing Wang, Kunming Liu, Jinbiao Liu. Construction Strategy and Imaging of Highly Selective Selenocysteine Fluorescent Probes [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 41-53. |
| [9] | Haoruo Shangguan, Ping Huang, Zhenya Dai, Ping Wang. Synthesis and Application of β-Thiolated Amino Acids [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3089-3097. |
| [10] | Huanqing Li, Zhaohua Chen, Zujia Chen, Qiwen Qiu, Youcai Zhang, Sihong Chen, Zhaoyang Wang. Research Progress in Mercury Ion Fluorescence Probes Based on Organic Small Molecules [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3067-3077. |
| [11] | Zhangtao Zhou, Yang Wang, Bingxin Cheng, Weiping Ye. [RuCl(p-cymene)-(S)-BINAP]Cl Catalyzed Asymmetric Preparation of trans-3-Amino-bicyclo[2.2.2]octane-2-carboxylic Acid Ethyl Ester [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2961-2967. |
| [12] | Binghui Ding, Shaohui Han, Haiqing Xiong, Benhua Wang, Bojun Zuo, Xiangzhi Song. A Highly Selective Ratiometric Fluorescent Probe for the Detection of Hypochlorite in Acute Lung Injury [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2878-2884. |
| [13] | Sifan Dong, Haolong Li, Yuan Qin, Shiming Fan, Shouxin Liu. Research Progress of Amino Acids as Transient Directing Groups in C—H Bond Activation Reactions [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2351-2367. |
| [14] | Tiantian Liu, Hongpeng Zhang, Xiaomeng Jiao, Yinjuan Bai. Research Progress of Multi-signal Fluorescent Probes for Simultaneous Detection of Biothiols [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2081-2095. |
| [15] | Yifang Li, Yao Wang, Huawei Niu, Xiujin Chen, Zhaozhou Li, Yongguo Wang. Research Progress of Sulfur Dioxide Fluorescent Probe Targeting Mitochondria [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 1952-1962. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||