Chinese Journal of Organic Chemistry ›› 2024, Vol. 44 ›› Issue (12): 3771-3783.DOI: 10.6023/cjoc202404004 Previous Articles Next Articles
ARTICLE
王崔颖b, 张颖b, 郑家练b, 袁佳a,b,*( ), 孟思璇b, 陈建b, 余广鳌b,*(
), 孟思璇b, 陈建b, 余广鳌b,*( )
)
收稿日期:2024-04-02
修回日期:2024-05-16
发布日期:2024-06-24
基金资助:
Cuiying Wangb, Ying Zhangb, Jia-Lian Zhengb, Jia Yuana,b,*( ), Sixuan Mengb, Jian Chenb, Guang-Ao Yub,*(
), Sixuan Mengb, Jian Chenb, Guang-Ao Yub,*( )
)
Received:2024-04-02
Revised:2024-05-16
Published:2024-06-24
Contact:
*E-mail:Supported by:Share
Cuiying Wang, Ying Zhang, Jia-Lian Zheng, Jia Yuan, Sixuan Meng, Jian Chen, Guang-Ao Yu. Efficient Construction of Chiral Binaphthalene-Core Phosphepine Compounds Using Palladium-Catalyzed C—P Coupling Reactions[J]. Chinese Journal of Organic Chemistry, 2024, 44(12): 3771-3783.
| Entry | Ligand | Base | Time/h | T/℃ | Conversionb/% |
|---|---|---|---|---|---|
| 1 | dppf | tBuONa | 16 | 110 | 85 |
| 2 | dippf | tBuONa | 16 | 110 | 100 |
| 3 | dippf | Cs2CO3 | 16 | 110 | 6 |
| 4 | dippf | K3PO4 | 16 | 110 | 17 |
| 5 | dippf | KF | 16 | 110 | 15 |
| 6 | dippf | tBuONa | 8 | 110 | 48 |
| 7 | dippf | tBuONa | 72 | 50 | 19 |
| 8 | No | tBuONa | 16 | 110 | 50 |
| 9c | dippf | tBuONa | 16 | 110 | 100 (91d, 97% ee) |
| Entry | Ligand | Base | Time/h | T/℃ | Conversionb/% |
|---|---|---|---|---|---|
| 1 | dppf | tBuONa | 16 | 110 | 85 |
| 2 | dippf | tBuONa | 16 | 110 | 100 |
| 3 | dippf | Cs2CO3 | 16 | 110 | 6 |
| 4 | dippf | K3PO4 | 16 | 110 | 17 |
| 5 | dippf | KF | 16 | 110 | 15 |
| 6 | dippf | tBuONa | 8 | 110 | 48 |
| 7 | dippf | tBuONa | 72 | 50 | 19 |
| 8 | No | tBuONa | 16 | 110 | 50 |
| 9c | dippf | tBuONa | 16 | 110 | 100 (91d, 97% ee) |
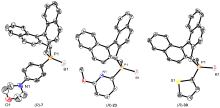

| [1] |
(a) Gladiali S.; Alberico E.; Junge K.; Beller M. Chem. Soc. Rev. 2011, 40, 3744.
|
|
(b) Lu D.; Salem G. Coord. Chem. Rev. 2013, 257, 1026.
|
|
|
(c) Fu W.; Tang W. ACS Catal. 2016, 6, 4814.
|
|
|
(d) Ni H.; Chan W.-L.; Lu Y. Chem. Rev. 2018, 118, 9344.
|
|
|
(e) Ye F.; Xu Z.; Xu L.-W. Acc. Chem. Res. 2021, 54, 452.
|
|
|
((f) Zhao W.; Yang D.; Zhang Y. Chin. J. Org. Chem. 2016, 36, 2301 (in Chinese).
|
|
|
(赵文献, 杨代月, 张玉华, 有机化学, 2016, 36, 2301.)
doi: 10.6023/cjoc201603006 |
|
| [2] |
(a) Xiao D.; Zhang Z.; Zhang X. Org. Lett. 1999, 1, 1679.
pmid: 23794450 |
|
(b) Junge K.; Oehme G.; Monsees A.; Riermeier T.; Dingerdissen U.; Beller M. Tetrahedron Lett. 2002, 43, 4977.
pmid: 23794450 |
|
|
(c) Junge K.; Wendt B.; Addis D.; Zhou S.; Das S.; Fleischer S.; Beller M. Chem.-Eur. J. 2011, 17, 101.
pmid: 23794450 |
|
|
(d) Jiang F.; Yuan K.; Achard M.; Bruneau C. Chem.-Eur. J. 2013, 19, 10343.
doi: 10.1002/chem.201301201 pmid: 23794450 |
|
|
(e) Humbert N.; Larionov E.; Mantilli L.; Nareddy P.; Besnard C.; Guénée L.; Mazet C. Chem.-Eur. J. 2014, 20, 745.
pmid: 23794450 |
|
| [3] |
(a) Monney A.; Alberico E.; Ortin Y.; Müller-Bunz H.; Gladiali S.; Albrecht M. Dalton Trans. 2012, 41, 8813.
|
|
(b) Bähr S.; Oestreich M. Organometallics 2017, 36, 935.
|
|
| [4] |
(a) Corkey B. K.; Toste F. D. J. Am. Chem. Soc. 2007, 129, 2764.
|
|
(b) Brissy D.; Skander M.; Jullien H.; Retailleau P.; Marinetti A. Org. Lett. 2009, 11, 2137.
|
|
|
(c) Nareddy P.; Mantilli L.; Guénée L.; Mazet C. Angew. Chem. Int. Ed. 2012, 51, 3826.
|
|
|
(d) Holstein P. M.; Vogler M.; Larini P.; Pilet G.; Clot E.; Baudoin O. ACS Catal. 2015, 5, 4300.
|
|
|
(e) Yue Q.; Liu B.; Liao G.; Shi B.-F. ACS Catal. 2022, 12, 9359.
|
|
| [5] |
(a) Luo H.-K.; Woo Y.-L.; Schumann H.; Jacob C.; Meurs M.; Yang H.-Y.; Tan Y.-T. Adv. Synth. Catal. 2010, 352, 1356.
pmid: 22216403 |
|
(b) Fujiwara Y.; Sun J.; Fu G. C. Chem. Sci. 2011, 2, 2196.
pmid: 22216403 |
|
| [6] |
(a) Fujiwara Y.; Fu G. C. J. Am. Chem. Soc. 2011, 133, 12293.
doi: 10.1021/ja2049012 pmid: 35404624 |
|
(b) Lee S. Y.; Fujiwara Y.; Nishiguchi A.; Kalek M.; Fu G. C. J. Am. Chem. Soc. 2015, 137, 4587.
pmid: 35404624 |
|
|
(c) Duan M.; Zhu L.; Qi X.; Yu Z.; Li Y.; Bai R.; Lan Y. Sci. Rep.-UK 2017, 7, 7619.
pmid: 35404624 |
|
|
(d) Maddigan-Wyatt J. T.; Cao J.; Ametovski J.; Hooper J. F.; Lupton D. W. Org. Lett. 2022, 24, 2847.
doi: 10.1021/acs.orglett.2c00785 pmid: 35404624 |
|
| [7] |
(a) Wurz R. P.; Fu G. C. J. Am. Chem. Soc. 2005, 127, 12234.
|
|
(b) Li Y.; Chen X.; Chen X.; Shen X. Molecules 2018, 23, 3022.
|
|
|
(c) Li K.; Gan Z.; Li E.-Q.; Duan Z. Org. Lett. 2021, 23, 3094.
|
|
| [8] |
(a) Mondal M.; Ibrahim A. A.; Wheeler K. A.; Kerrigan N. J. Org. Lett. 2010, 12, 1664.
pmid: 22409456 |
|
(b) Chen S.; Salo E. C.; Wheeler K. A.; Kerrigan N. J. Org. Lett. 2012, 14, 1784.
doi: 10.1021/ol3003783 pmid: 22409456 |
|
| [9] |
(a) Sinisi R.; Sun J.; Fu G. C. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 20652.
pmid: 22216403 |
|
(b) Fujiwara Y.; Sun J.; Fu G. C. Chem. Sci. 2011, 2, 2196.
pmid: 22216403 |
|
|
(c) Kalek M.; Fu G. C. J. Am. Chem. Soc. 2015, 137, 9438.
pmid: 22216403 |
|
|
((d) Yu Y.-N.; Xu M.-H. Acta Chim. Sinica 2014, 72, 815 (in Chinese).
pmid: 22216403 |
|
|
(于月娜, 徐明华, 化学学报, 2014, 72, 815.)
doi: 10.6023/A14060436 pmid: 22216403 |
|
|
((e) Wan B.; Zhao Q.; Wang L. Chin. J. Catal. 2010, 31, 514 (in Chinese).
pmid: 22216403 |
|
|
(万博, 赵庆鲁, 王来来, 催化学报, 2010, 31, 514.)
pmid: 22216403 |
|
| [10] |
Gladiali S.; Dore A.; Fabbri D.; De Lucchi O.; Manassero M. Tetrahedron: Asymmetry 1994, 5, 511.
|
| [11] |
Gulyás B.; Rivilla I.; Curreli S.; Freixa Z.; Leeuwen P. W. N. M. Catal. Sci. Technol. 2015, 5, 3822.
|
| [12] |
(a) Bitterer F.; Herd O.; Ku1hnel M.; Stelzer O.; Weferling N.; Sheldrick W. S.; Hahn J.; Nagel S.; Rö1sch N. Inorg. Chem. 1998, 37, 6408.
pmid: 11670760 |
|
(b) Huang Z.; Xie J. Chin. J. Org. Chem. 2023, 43, 4308 (in Chinese).
pmid: 11670760 |
|
|
(黄争艳, 谢建华, 有机化学, 2023, 43, 4308.)
doi: 10.6023/cjoc202300068 pmid: 11670760 |
|
| [13] |
Maigrot N.; Mazaleyrat J. P. Synthesis 1985, 317.
|
| [14] |
(a) Scriban C.; Glueck D. S. J. Am. Chem. Soc. 2006, 128, 2788.
pmid: 18808136 |
|
(b) Chan V. S.; Chiu M.; Bergman R. G.; Toste F. D. J. Am. Chem. Soc. 2009, 131, 6021.
pmid: 18808136 |
|
|
(c) Anderson B. J.; Reynolds S. C.; Guino-o M. A.; Xu Z.; Glueck D. S. ACS Catal. 2016, 6, 8106.
pmid: 18808136 |
|
|
(d) Chan V. S; Stewart I. C.; Bergman R. G.; Toste F. D. J. Am. Chem. Soc. 2006, 128, 2786.
pmid: 18808136 |
|
|
(e) Anderson B. J.; Guino-o M. A.; Glueck D. S.; Golen J. A.; DiPasquale A. G.; Liable-Sands L. M.; Rheingold A. L. Org. Lett. 2008, 10, 4425.
doi: 10.1021/ol801616s pmid: 18808136 |
|
|
(f) Luan C.; Yang C.-J.; Liu L.; Gu Q.-S.; Liu X.-Y. Chem. Catal. 2022, 2, 2876.
pmid: 18808136 |
|
|
(g) Kang J.; Ding K.; Ren S.-M.; Su B. Angew. Chem., Int. Ed. 2023, 62, e202301628.
pmid: 18808136 |
|
| [15] |
(a) Liu X.-T.; Zhang Y.-Q.; Han X.-Y.; Sun S.-P.; Zhang Q.-W. J. Am. Chem. Soc. 2019, 141, 16584.
|
|
(b) Li C.; Yang S. Chin. J. Org. Chem. 2020, 40, 2159 (in Chinese).
|
|
|
(李冲, 杨尚东, 有机化学, 2020, 40, 2159.)
doi: 10.6023/cjoc202000037 |
|
|
(c) Jia S.; Chen S.; Liu Z.; Cheng H.; Zhou Q. Chin. J. Org. Chem. 2022, 42, 3373 (in Chinese).
|
|
|
(贾仕虎, 陈思元, 刘泽水, 程鸿刚, 周强辉, 有机化学, 2022, 42, 3373.)
doi: 10.6023/cjoc202209002 |
|
| [16] |
(a) Moncarz J. R.; Laritcheva N. F.; Glueck D. S. J. Am. Chem. Soc. 2002, 124, 13356.
pmid: 17298077 |
|
(b) Chan V. S.; Bergman R. G.; Toste F. D. J. Am. Chem. Soc. 2007, 129, 15122.
pmid: 17298077 |
|
|
(c) Blank N. F.; Moncarz J. R.; Brunker T. J.; Scriban C.; Anderson B. J.; Amir O.; Glueck D. S.; Zakharov L. N.; Golen J. A.; Incarvito C. D.; Rheingold A. L. J. Am. Chem. Soc. 2007, 129, 6847.
pmid: 17298077 |
|
|
(d) Brunker T. J.; Anderson B. J.; Blank N. F.; Glueck D. S.; Rheingold A. L. Org. Lett. 2007, 9, 1109.
pmid: 17298077 |
|
|
(e) Lin Z.-Q.; Wang W.-Z.; Yan S.-B.; Duan W.-L. Angew. Chem., Int. Ed. 2015, 54, 6265.
pmid: 17298077 |
|
|
(f) Beaud R.; Phipps R. J.; Gaunt M. J. J. Am. Chem. Soc. 2016, 138, 13183.
pmid: 17298077 |
|
|
(g) Dai Q.; Li W.; Li Z.; Zhang J. J. Am. Chem. Soc. 2019, 141, 20556.
pmid: 17298077 |
|
| [17] |
Wang C.; Yue C.-D.; Yuan J.; Zheng J.-L.; Zhang Y.; Yu H.; Chen J.; Meng S.; Yu Y.; Yu G.-A.; Che C.-M. Chem. Commun. 2020, 56, 11775.
|
| [18] |
(a) Sadow A. D.; Haller I.; Fadini L.; Togni A. J. Am. Chem. Soc. 2004, 126, 14704.
|
|
(b) Sadow A. D.; Togni A. J. Am. Chem. Soc. 2005, 127, 17012.
|
|
|
(c) Feng J.-J.; Chen X.-F.; Shi M.; Duan W.-L. J. Am. Chem. Soc. 2010, 132, 5562.
|
|
|
(d) Nie S.-Z.; Davison R. T.; Dong V. M. J. Am. Chem. Soc. 2018, 140, 16450.
|
|
|
(e) Lu Z.; Zhang H.; Yang Z.; Ding N.; Meng L.; Wang J. ACS Catal. 2019, 9, 1457.
|
|
|
(f) Wang C.; Yang Q.; Dai Y.-H.; Xiong J.; Zheng Y.; Duan W.-L. Angew. Chem., Int. Ed. 2023, 62, e202313112.
|
| [1] | Yuxuan Yan, Wanqing Lu, Huijun Qian, Leiyang Lv, Zhiping Li. Pd-Catalyzed Ring-Opening of gem-Difluorocyclopropanes for the Mono- and Bis-fluoroallylation of 1,3-Dicarbonyls [J]. Chinese Journal of Organic Chemistry, 2024, 44(5): 1630-1640. |
| [2] | Fakai Zou, Nengzhong Wang, Hui Yao, Hui Wang, Mingguo Liu, Nianyu Huang. Regio- and Stereo-selective Synthesis of 1β-/3R-Aryl Thiosugar [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 593-604. |
| [3] | Xun Xiang, Zhaolin He, Xiuqin Dong. Recent Advances of Efficient Synthesis of Chiral Molecules Promoted by Pd/Chiral Phosphoric Acid Synergistic Catalysis [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 791-808. |
| [4] | Yubing Shi, Wenji Bai, Weihua Mu, Jiangping Li, Jiawei Yu, Bing Lian. Research Progress on Density Functional Theory Study of Palladium-Catalyzed C—H Functionalization to Form C—X (X=O, N, F, I, …) Bonds [J]. Chinese Journal of Organic Chemistry, 2022, 42(5): 1346-1374. |
| [5] | Ma Xiantao, Yu Jing, Wang Zilong, Zhang Yun, Zhou Qiuju. Efficient Activation of Allylic Alcohols in Pd-Catalyzed Allylic Substitution Reactions [J]. Chinese Journal of Organic Chemistry, 2020, 40(9): 2669-2677. |
| [6] | Bian Rongjian, Bao Xiaoguang. Computational Insights into the Mechanism of Pd(0) and Ben-zoic Acid Co-Catalyzed Hydroamination of Internal Alkynes [J]. Chin. J. Org. Chem., 2017, 37(1): 190-195. |
| [7] | Zheng Longsheng, Song Tao, Xu Liwen. 1,1’-Binaphthalene-2-α-arylmethanol-2’-ols (Ar-BINMOLs) with Axial and sp3-Central Chirality:A Novel Chiral Ligands for Catalytic Asymmetric Transformations [J]. Chin. J. Org. Chem., 2014, 34(7): 1255-1267. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||