Chinese Journal of Organic Chemistry ›› 2025, Vol. 45 ›› Issue (1): 276-285.DOI: 10.6023/cjoc202405010 Previous Articles Next Articles
ARTICLES
路星星a, 林誉凡a, 徐欢a, 张晓鸣a,*( ), 李雪生b, 凌云a, 杨新玲a,*(
), 李雪生b, 凌云a, 杨新玲a,*( )
)
收稿日期:2024-06-21
修回日期:2024-07-24
发布日期:2024-08-16
基金资助:
Xingxing Lua, Yufan Lina, Huan Xua, Xiaoming Zhanga( ), Xuesheng Lib, Yun Linga, Xinling Yanga(
), Xuesheng Lib, Yun Linga, Xinling Yanga( )
)
Received:2024-06-21
Revised:2024-07-24
Published:2024-08-16
Contact:
*E-mail: Supported by:Share
Xingxing Lu, Yufan Lin, Huan Xu, Xiaoming Zhang, Xuesheng Li, Yun Ling, Xinling Yang. Design, Synthesis and Bioactivity Research of Novel Bipyridine Compounds[J]. Chinese Journal of Organic Chemistry, 2025, 45(1): 276-285.
| Compd. | 200 mg•L-1 | 100 mg•L-1 |
|---|---|---|
| T1 | 100 | 80.96±5.42 |
| T2 | 95.70±2.72 | 75.48±5.53 |
| T3 | 71.32±6.77 | |
| T4 | 82.80±5.93 | 12.77±2.41 |
| T5 | 59.14±7.58 | |
| T6 | 81.91±4.10 | 2.06±2.39 |
| T7 | 78.49±7.20 | |
| T8 | 95.80±1.33 | 4.62±0.66 |
| T9 | 62.72±10.22 | |
| T10 | 95.70±2.72 | 23.91±0.68 |
| T11 | 19.61±7.58 | |
| T12 | 3.92±3.60 | |
| T13 | 46.43±1.83 | 37.84±2.19 |
| T14 | 79.70±2.24 | |
| T15 | 33.64±6.22 | |
| T16 | 70.85±2.34 | |
| 3c | 72.09±3.14 | 41.67±6.80 |
| FLP | 100 | 100 |
| Pymetrozine | 86.64±7.05 | 76.13±4.80 |
| Compd. | 200 mg•L-1 | 100 mg•L-1 |
|---|---|---|
| T1 | 100 | 80.96±5.42 |
| T2 | 95.70±2.72 | 75.48±5.53 |
| T3 | 71.32±6.77 | |
| T4 | 82.80±5.93 | 12.77±2.41 |
| T5 | 59.14±7.58 | |
| T6 | 81.91±4.10 | 2.06±2.39 |
| T7 | 78.49±7.20 | |
| T8 | 95.80±1.33 | 4.62±0.66 |
| T9 | 62.72±10.22 | |
| T10 | 95.70±2.72 | 23.91±0.68 |
| T11 | 19.61±7.58 | |
| T12 | 3.92±3.60 | |
| T13 | 46.43±1.83 | 37.84±2.19 |
| T14 | 79.70±2.24 | |
| T15 | 33.64±6.22 | |
| T16 | 70.85±2.34 | |
| 3c | 72.09±3.14 | 41.67±6.80 |
| FLP | 100 | 100 |
| Pymetrozine | 86.64±7.05 | 76.13±4.80 |
| Compd. | y=ax+b | LC50/ (mg•L-1) | 95% confiden- ce interval | r |
|---|---|---|---|---|
| T1 | y=1.17x-1.23 | 11.04 | 6.38~15.65 | 0.98 |
| T2 | y=0.74x-1.00 | 25.12 | 16.97~35.77 | 0.99 |
| 3c | y=1.85x-3.71 | 102.38 | 87.35~123.14 | 0.97 |
| Pymetrozine | y=0.70x-0.54 | 5.85 | 2.51~9.78 | 0.98 |
| FLP | y=0.84x+0.61 | 0.20 | 0.12~0.29 | 0.98 |
| Compd. | y=ax+b | LC50/ (mg•L-1) | 95% confiden- ce interval | r |
|---|---|---|---|---|
| T1 | y=1.17x-1.23 | 11.04 | 6.38~15.65 | 0.98 |
| T2 | y=0.74x-1.00 | 25.12 | 16.97~35.77 | 0.99 |
| 3c | y=1.85x-3.71 | 102.38 | 87.35~123.14 | 0.97 |
| Pymetrozine | y=0.70x-0.54 | 5.85 | 2.51~9.78 | 0.98 |
| FLP | y=0.84x+0.61 | 0.20 | 0.12~0.29 | 0.98 |
| Compd. | Pythium aphanidermatum | Magnaporthe oryzae | Valsa mali | Botrytis cinerea | Phytophthora capsici |
|---|---|---|---|---|---|
| T1 | 7.42±3.24 | 19.62±1.48 | 8.42±0.74 | 45.65±2.91 | 8.03±0 |
| T2 | 16.79±0.65 | 25.77±0.33 | 6.57±1.32 | 47.39±1.43 | 14.72±0.84 |
| T3 | 49.03±0.75 | 31.15±0.33 | 2.88±0.61 | 44.35±0.87 | 32.69±0.36 |
| T4 | 77.51±1.84 | 43.85±1.92 | 9.53±1.68 | 93.48±0.38 | 45.65±1.25 |
| T5 | 52.77±4.90 | 70.38±4.19 | 16.54±1.11 | 100 | 34.78±0.84 |
| T6 | 11.54±1.91 | 64.23±2.39 | 38.70±1.11 | 91.30±0 | 14.30±0.36 |
| T7 | 63.27±2.02 | 27.69±0.54 | 9.16±1.11 | 40.87±0.61 | 41.47±0 |
| T8 | 63.27±0.65 | 32.69±3.28 | 15.07±1.85 | 40.00±1.44 | 49.83±0 |
| T9 | 35.53±1.50 | 31.92±0.64 | 26.14±5.17 | 47.83±2.13 | 50.25±0.36 |
| T10 | 7.05±1.59 | 45.38±1.15 | 49.04±0.64 | 60.00±0.61 | 44.40±0.36 |
| T11 | 3.30±0.65 | 23.85±0.38 | 22.82±0.32 | 38.70±5.08 | 16.81±0.36 |
| T12 | 5.55±1.91 | 36.15±1.28 | 30.21±1.09 | 50.00±0.38 | 23.49±0.69 |
| T13 | 40.78±2.73 | 40.00±1.44 | 32.42±0.61 | 46.09±0.87 | 31.86±0.36 |
| T14 | 38.91±5.26 | 20.38±1.00 | 14.33±0 | 46.09±0.87 | 17.22±1.25 |
| T15 | 25.04±5.00 | 31.15±3.66 | 6.20±1.11 | 40.00±0.75 | 18.06±0.59 |
| T16 | 40.40±3.53 | 78.46±3.88 | 36.85±2.05 | 46.96±0.75 | 33.95±0.42 |
| 3c | 13.97±0.83 | 54.02±0.50 | 52.86±0.65 | 47.66±0.75 | 25.30±0.75 |
| Pyraclostrobin | 100 | 100 | 100 | 24.35±1.30 | 4.68±0 |
| Prothioconazole | 57.77±1.48 | 100 | 100 | 100 | 66.76±1.12 |
| Compd. | Pythium aphanidermatum | Magnaporthe oryzae | Valsa mali | Botrytis cinerea | Phytophthora capsici |
|---|---|---|---|---|---|
| T1 | 7.42±3.24 | 19.62±1.48 | 8.42±0.74 | 45.65±2.91 | 8.03±0 |
| T2 | 16.79±0.65 | 25.77±0.33 | 6.57±1.32 | 47.39±1.43 | 14.72±0.84 |
| T3 | 49.03±0.75 | 31.15±0.33 | 2.88±0.61 | 44.35±0.87 | 32.69±0.36 |
| T4 | 77.51±1.84 | 43.85±1.92 | 9.53±1.68 | 93.48±0.38 | 45.65±1.25 |
| T5 | 52.77±4.90 | 70.38±4.19 | 16.54±1.11 | 100 | 34.78±0.84 |
| T6 | 11.54±1.91 | 64.23±2.39 | 38.70±1.11 | 91.30±0 | 14.30±0.36 |
| T7 | 63.27±2.02 | 27.69±0.54 | 9.16±1.11 | 40.87±0.61 | 41.47±0 |
| T8 | 63.27±0.65 | 32.69±3.28 | 15.07±1.85 | 40.00±1.44 | 49.83±0 |
| T9 | 35.53±1.50 | 31.92±0.64 | 26.14±5.17 | 47.83±2.13 | 50.25±0.36 |
| T10 | 7.05±1.59 | 45.38±1.15 | 49.04±0.64 | 60.00±0.61 | 44.40±0.36 |
| T11 | 3.30±0.65 | 23.85±0.38 | 22.82±0.32 | 38.70±5.08 | 16.81±0.36 |
| T12 | 5.55±1.91 | 36.15±1.28 | 30.21±1.09 | 50.00±0.38 | 23.49±0.69 |
| T13 | 40.78±2.73 | 40.00±1.44 | 32.42±0.61 | 46.09±0.87 | 31.86±0.36 |
| T14 | 38.91±5.26 | 20.38±1.00 | 14.33±0 | 46.09±0.87 | 17.22±1.25 |
| T15 | 25.04±5.00 | 31.15±3.66 | 6.20±1.11 | 40.00±0.75 | 18.06±0.59 |
| T16 | 40.40±3.53 | 78.46±3.88 | 36.85±2.05 | 46.96±0.75 | 33.95±0.42 |
| 3c | 13.97±0.83 | 54.02±0.50 | 52.86±0.65 | 47.66±0.75 | 25.30±0.75 |
| Pyraclostrobin | 100 | 100 | 100 | 24.35±1.30 | 4.68±0 |
| Prothioconazole | 57.77±1.48 | 100 | 100 | 100 | 66.76±1.12 |
| Compd. | y=ax+b | EC50/ (mg•L-1) | 95% confiden- ce interval | r |
|---|---|---|---|---|
| T4 | y=2.15x-2.60 | 17.50 | 12.07~27.93 | 0.95 |
| T5 | y=2.22x-1.93 | 7.45 | 5.79~9.53 | 0.98 |
| T6 | y=1.77x-1.78 | 10.54 | 9.08~12.29 | 0.98 |
| Prothioconazole | y=0.85x-0.59 | 4.90 | 3.52~7.09 | 0.99 |
| Compd. | y=ax+b | EC50/ (mg•L-1) | 95% confiden- ce interval | r |
|---|---|---|---|---|
| T4 | y=2.15x-2.60 | 17.50 | 12.07~27.93 | 0.95 |
| T5 | y=2.22x-1.93 | 7.45 | 5.79~9.53 | 0.98 |
| T6 | y=1.77x-1.78 | 10.54 | 9.08~12.29 | 0.98 |
| Prothioconazole | y=0.85x-0.59 | 4.90 | 3.52~7.09 | 0.99 |
| Compd. | Mutagenisis | Carcinogenicity | Bee toxicity | Acute oral toxicity in rats | |
|---|---|---|---|---|---|
| LD50/(mg•kg-1) | Toxicity grade (US EPA)a | ||||
| T1 | None | None | Low | 637.85 | Low |
| T2 | None | None | Low | 522.07 | Low |
| T3 | None | None | Low | 436.35 | Low |
| T4 | None | None | Low | 451.86 | Low |
| T5 | None | None | Low | 527.16 | Low |
| T6 | None | None | Low | 712.76 | Low |
| T7 | None | None | Low | 752.48 | Low |
| T8 | None | None | Low | —b | |
| T9 | None | None | Low | 555.54 | Low |
| T10 | None | None | Low | 357.33 | Moderate |
| T11 | None | None | Low | 1087.59 | Low |
| T12 | None | None | Low | 993.15 | Low |
| T13 | None | None | Low | 1067.69 | Low |
| T14 | Low | High | Low | 564.57 | Low |
| T15 | None | None | Low | 806.12 | Low |
| T16 | None | None | Low | 2454.79 | Low |
| Compd. | Mutagenisis | Carcinogenicity | Bee toxicity | Acute oral toxicity in rats | |
|---|---|---|---|---|---|
| LD50/(mg•kg-1) | Toxicity grade (US EPA)a | ||||
| T1 | None | None | Low | 637.85 | Low |
| T2 | None | None | Low | 522.07 | Low |
| T3 | None | None | Low | 436.35 | Low |
| T4 | None | None | Low | 451.86 | Low |
| T5 | None | None | Low | 527.16 | Low |
| T6 | None | None | Low | 712.76 | Low |
| T7 | None | None | Low | 752.48 | Low |
| T8 | None | None | Low | —b | |
| T9 | None | None | Low | 555.54 | Low |
| T10 | None | None | Low | 357.33 | Moderate |
| T11 | None | None | Low | 1087.59 | Low |
| T12 | None | None | Low | 993.15 | Low |
| T13 | None | None | Low | 1067.69 | Low |
| T14 | Low | High | Low | 564.57 | Low |
| T15 | None | None | Low | 806.12 | Low |
| T16 | None | None | Low | 2454.79 | Low |
| Compd. | Mortality rate/% | Bee toxicitya | |
|---|---|---|---|
| Oral toxicity | Contact toxicity | ||
| Blank control | 10.00 | 10.00 | |
| Solvent control | 3.33 | 6.67 | |
| T1b | 0.00 | 3.33 | Low |
| Pymetrozine | LD50>60 μg/bee | LD50>12 μg/bee | Low |
| Imidacloprid | LD50=2.82×10-2 μg/bee | LD50=5.78×10-2 μg/bee | High |
| Compd. | Mortality rate/% | Bee toxicitya | |
|---|---|---|---|
| Oral toxicity | Contact toxicity | ||
| Blank control | 10.00 | 10.00 | |
| Solvent control | 3.33 | 6.67 | |
| T1b | 0.00 | 3.33 | Low |
| Pymetrozine | LD50>60 μg/bee | LD50>12 μg/bee | Low |
| Imidacloprid | LD50=2.82×10-2 μg/bee | LD50=5.78×10-2 μg/bee | High |
| Ligand | ΔΕvdw/(kcal•mol-1) | ΔEele/(kcal•mol-1) | ΔGgas/(kcal•mol-1) | ΔGsol/(kcal•mol-1) | ΔGbind/(kcal•mol-1) |
|---|---|---|---|---|---|
| T1 | -31.03±2.33 | -14.50±1.36 | -46.53±2.94 | 22.05±1.10 | -24.48±2.90 |
| 3c | -38.12±2.08 | -8.41±8.22 | -46.52±9.16 | 22.83±5.08 | -23.69±4.58 |
| Ligand | ΔΕvdw/(kcal•mol-1) | ΔEele/(kcal•mol-1) | ΔGgas/(kcal•mol-1) | ΔGsol/(kcal•mol-1) | ΔGbind/(kcal•mol-1) |
|---|---|---|---|---|---|
| T1 | -31.03±2.33 | -14.50±1.36 | -46.53±2.94 | 22.05±1.10 | -24.48±2.90 |
| 3c | -38.12±2.08 | -8.41±8.22 | -46.52±9.16 | 22.83±5.08 | -23.69±4.58 |
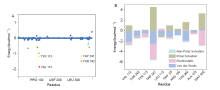
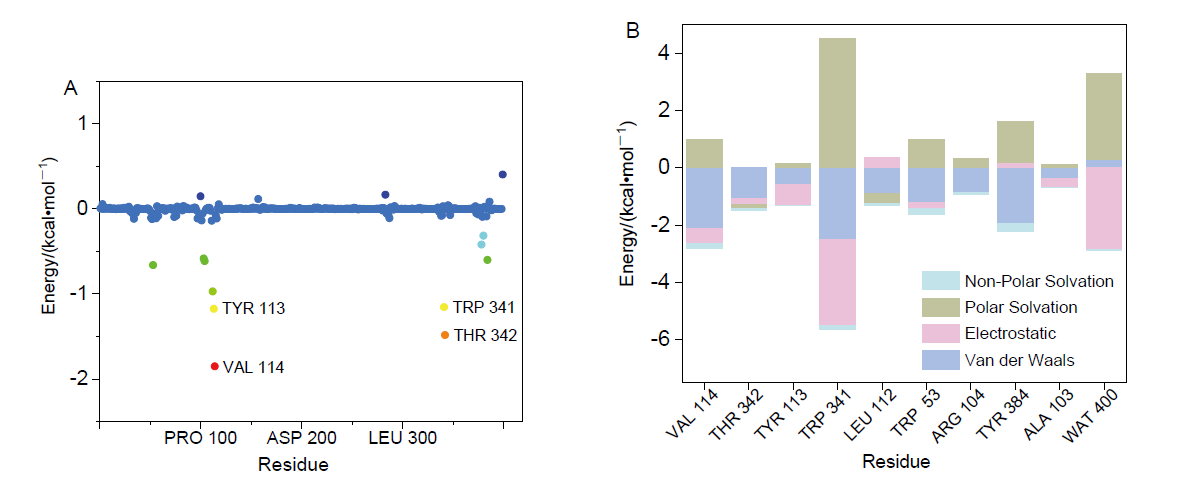
| [1] |
Li, D.-K.; Zheng, Q.-C.; Li, Z.-Y.; Xie, Y.; Zhang, L.-X.; Zhao, L.-K.; Yang, Y.; Liu, J.; Na, R.-S. Agrochemicals 2022, 61, 10 (in Chinese).
|
|
(李登奎, 郑乾成, 李志愿, 谢勇, 张立新, 赵利康, 杨莹, 刘佳, 那日松, 农药, 2022, 61, 10.)
|
|
| [2] |
Tsukamoto, M.; Nakamura, T.; Kimura, H.; Nakayama, H. J. Pestic. Sci. 2021, 46, 2.
|
| [3] |
DeBoer, G. J.; Thornburgh, S.; Gilbert, J.; Gast, R. E. Pest Manage. Sci. 2011, 67, 3.
|
| [4] |
Edmunds, A. J. F. Chimia 2022, 76, 7.
|
| [5] |
Epp, J. B.; Schmitzer, P. R.; Crouse, G. D. Pest Manage. Sci. 2018, 74, 1.
|
| [6] |
Li, Y.-W.; Tang, Y.-D.; Xue, Z.-W.; Wang, Y.; Shi, Y.-F.; Gao, X.-H.; Li, X.; Li, G.-X.; Li, F.; Lu, L.; Miao, J.-Q.; Liu, X.-L. J. Agric. Food Chem. 2023, 71, 4.
|
| [7] |
Babij, N. R.; Choy, N.; Cismesia, M. A.; Couling, D. J.; Hough, N. M.; Johnson, P. L.; Klosin, J.; Li, X.-Y.; Lu, Y.; McCusker, E. O.; Meyer, K. G.; Renga, J. M.; Rogers, R. B.; Stockman, K. E.; Webb, N. J.; Whiteker, G. T.; Zhu, Y.-M. Green Chem. 2020, 22, 18.
|
| [8] |
Jackson, K. L.; Yin, J.-F.; Csinos, A. S.; Ji, P.-S. Crop Prot. 2010, 29, 12.
|
| [9] |
Royals, B.; Brandenburg, R.; Hare, A.; Jordan, D.; Taylor, S.; Malone, S. Crop, Forage Turfgrass Manage. 2020, 6, 1.
|
| [10] |
Lahm, G.P.; Cordova, D.; Barry, J. D. Bioorg. Med. Chem. 2009, 17, 12.
|
| [11] |
Sheng, Z.-B.; Wang, J.; Pei, H.-Y.; Gao, Y.-X.; Zhang, J.; Zhang, L.-X. Agrochemicals 2021, 60, 1 (in Chinese).
|
|
(盛祝波, 汪杰, 裴鸿艳, 高一星, 张静, 张立新, 农药, 2021, 60, 1.)
|
|
| [12] |
Tan, H.-J. World Agrochem. 2019, 41, 5 (in Chinese).
|
|
(谭海军, 世界农药, 2019, 41, 5.)
|
|
| [13] |
Kleier, D.; Holden, I.; Casida, J. E.; Ruzo, L. O. J. Agric. Food Chem. 1985, 33, 5.
|
| [14] |
Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. J. Agric. Food Chem. 2011, 59, 7.
|
| [15] |
Casida, J. E.; Durkin, K. A. Annu. Rev. Entomol. 2013, 58, 99.
|
| [16] |
Nauen, R.; Jeschke, P.; Velten, R.; Beck, M. E.; Ebbinghaus- Kintscher, U.; Thielert, W.; Wölfel, K.; Haas, M.; Kunz, K.; Raupach, G. Pest Manage. Sci. 2015, 71, 6.
|
| [17] |
Onozaki, Y.; Horikoshi, R.; Ohno, I.; Kitsuda, S.; Durkin, K. A.; Suzuki, T.; Asahara, C.; Hiroki, N.; Komabashiri, R.; Shimizu, R.; Furutani, S.; Ihara, M.; Matsuda, K.; Mitomi, M.; Kagabu, S.; Uomoto, K.; Tomizawa, M. J. Agric. Food Chem. 2017, 65, 36.
|
| [18] |
Tan, H.-J. World Pestic. 2021, 43, 1 (in Chinese).
|
|
(谭海军, 世界农药, 2021, 43, 1.)
|
|
| [19] |
Lu, X.-X.; Xu, H.; Zhang, X.-M.; Sun, T.-D.; Lin, Y.-F.; Zhang, Y.-H.; Li, H.-H.; Li, X.-S.; Yang, X.-L.; Duan, H.-X.; Ling, Y. Molecules 2022, 27, 18.
|
| [20] |
Lu, X.-X.; Xu, H.; Zhang, X.-M.; Sun, T.-D.; Lin, Y.-F.; Li, H.-H.; Li, X.-S.; Zhang, L.; Duan, H.-X.; Yang, X.-L.; Ling, Y. J. Agric. Food Chem. 2023, 71, 49.
|
| [21] |
Yang, Z.-K.; Wu, X.; Zhang, J.-L.; Lu, X.-X.; Li, X.; Jiang, Z.-Y.; Song, D.-L.; Duan, H.-X.; Yang, X.-L. Chin. J. Org. Chem. 2021, 41, 7 (in Chinese).
|
|
(杨朝凯, 武霞, 张晋陆, 路星星, 李想, 蒋志洋, 宋敦伦, 段红霞, 杨新玲, 有机化学, 2021, 41, 7.)
|
|
| [22] |
Adriaanse, P.; Arce, A.; Focks, A.; Ingels, B.; Jölli, D.; Lambin, S.; Rundlöf, M.; Süßenbach, D.; Del Aguila, M.; Ercolano, V.; Ferilli, F.; Ippolito, A.; Szentes, C.; Neri, F. M.; Padovani, L.; Rortais, A.; Wassenberg, J.; Auteri, D. EFSA J. 2023, 21, 7989.
|
| [23] |
Bailey, J.; Scott-Dupree, C.; Harris, R.; Tolman, J.; Harris, B. Apidologie 2007, 36, 4.
|
| [24] |
Mommarets, V.; Sterk, G.; Smagghe, G. Pest Manage. Sci. 2006, 62, 8.
|
| [25] |
Oruc, H. H.; Hranitz, J. M.; Sorucu, A.; Duell, M.; Cakmak, I.; Aydin, L.; Orman, A. J. Econ. Entomol. 2012, 105, 6.
|
| [26] |
Talley, T. T.; Harel, M.; Hibbs, R. E.; Radić, Z.; Tomizawa, M.; Casida, J. E.; Taylor, P. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 21.
|
| [27] |
Elbaramawi, S. S.; Ibrahim, S. M.; Lashine, E. S. M.; El-Sadek, M. E.; Mantzourani, E.; Simons, C. J. Mol. Graphics Modell. 2017, 73, 36.
|
| [28] |
Case, D. A.; Cheatham, T. E.; Darden, T.; Gohlke, H.; Luo, R.; Merz, K. M.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R. J. J. Comput. Chem. 2005, 26, 16.
|
| [29] |
Maier, J. A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K. E.; Simmerling, C. J. Chem. Theory Comput. 2015, 11, 8.
|
| [30] |
Wang, J.; Wolf, R. M.; Caldwell, J. W.; Kollman, P. A.; Case, D. A. J. Comput. Chem. 2004, 25, 9.
|
| [1] | Haiping Tian, Dongdong Liu, Hongyan Pei, Jialin Ye, Zirui Zheng, Yixing Gao, Changxing Li, Huan Tian, Jing Zhang, Lixin Zhang. Design, Synthesis and Insecticidal Activity of New Phenylpyrazole Derivatives [J]. Chinese Journal of Organic Chemistry, 2025, 45(1): 227-239. |
| [2] | Jiyong Liu, Minghui Wu, Juncheng Xiang, Huailin Pang, Bin Li, Liang Lü. Synthesis, Insecticidal Activities, and Structure-Activity Relationship Studies of Novel Diamide Compounds Containing (Halogenated) Alkoxy Group [J]. Chinese Journal of Organic Chemistry, 2024, 44(5): 1584-1591. |
| [3] | Min Liu, Dongyan Yang, Yumei Xiao, Wangcang Su, Fenghai Zhao, Qin Zhaohai .. Synthesis and Bioactivities of 5-Nitroimino-[1,4-2H]-1,2,4-triazolines as Olefin-Imidacloprid Mimics [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2790-2799. |
| [4] | Zichan Zhang, Yang Sun, Sheng Hua, Baolin Xu, Min Zhang, Qin Zhao, Dandan Zheng, Yang Wang, Jianfeng Ju, Yujun Shi, Hong Dai. Synthesis and Insecticidal Activity of Novel Pyrazole Amides Containing an Isoxazole Moiety [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1435-1443. |
| [5] | Lei Wang, Shujing Yu, Na Yang, Baolei Wang. Studies on the Synthesis and Biological Activities of Novel Dihydroquinazolinone-Containing Caffeine Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 299-307. |
| [6] | Wenjuan Li, Rui Zhang, Zhihua Cai, Xiaoqiang Han, Lin He, Bin Dai. Constrution and Insecticidal Activities of Trifluoromethylated Benzocyclicsulfoximine Derivatives by [3+2] Cycloaddition Reaction of Beznyne [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2832-2839. |
| [7] | Shu Chen, Yingying Shao, Xinhao Fu, Qingwu Chen, Xiaohua Du, Chengxia Tan. Design, Synthesis and Insecticidal Activities of Pyridyl Thiazole Diamide Compounds [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3870-3879. |
| [8] | Ali Dai, Renfeng Zhang, Chuanhui Li, Lijiao Yu, Ya Wang, Jian Wu. Synthesis and Biological Activity of N-Cyano Sulfonimide Derivatives Bearing Trifluoromethyl Pyridinamide [J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3633-3642. |
| [9] | Feng He, Shengxin Guo, Ali Dai, Renfeng Zhang, Jian Wu. Synthesis, Characterization, and Biological Activity of Novel Amide Derivatives Containing Trifluoromethylpyridine Moieties [J]. Chinese Journal of Organic Chemistry, 2021, 41(8): 3303-3311. |
| [10] | Jinjiao Dong, Xinyue Zhu, Siran Feng, Chaochao Zhang, Zhenming Liu, Xiaoqiang Qiao, Yali Song. Synthesis and Antifungal Activity of 7-Phenyl-6H,7H-1,3,4-thia- diazolo[3,2-a]-thiochromeno[4,3-d]pyrimidine Compounds [J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2467-2475. |
| [11] | Hang Liu, Shuyun Zhang, Huan Li, Yan Zhang, Zhengming Li, Baolei Wang. Synthesis and Biological Activities of Novel 8-((3,4,4-Trifluorobut-3-en-1-yl)thio)-substituted Methylxanthines and Their Derivatives [J]. Chinese Journal of Organic Chemistry, 2021, 41(5): 2091-2098. |
| [12] | Yangyi Yao, Chaoli Ren, Li Chen, Liangkun Zhong, Tianming Xu, Chengxia Tan. Synthesis and Insecticidal Activity of 3-Ethyl Sulfone Pyridine Substituted Aryl Triazole Compounds [J]. Chinese Journal of Organic Chemistry, 2021, 41(5): 2055-2062. |
| [13] | Weibin Xie, Huangong Li, Xiangde Meng, Zhengming Li, Sha Zhou. Design, Synthesis and Biological Activity Study of Novel N-Phenylpyrazole Anthranilic Diamides Containing Heptafluoroisopropyl Moieties [J]. Chinese Journal of Organic Chemistry, 2021, 41(4): 1722-1727. |
| [14] | Shuyun Zhang, Hang Liu, Lei Wang, Zhengming Li, Baolei Wang. Studies on the Synthesis, Structural Characterization and Biological Activities of Novel Caffeine Derivatives Containing Substituted-Piperazine Moiety [J]. Chinese Journal of Organic Chemistry, 2021, 41(10): 4075-4082. |
| [15] | Zhang Jingpeng, Yang Zhaokai, Qin Yaoguo, Yang Xinling. Design, Synthesis and Biological Activity of (E)-β-Farnesene Analogues Containing 1,2,3-Thiadiazole [J]. Chinese Journal of Organic Chemistry, 2020, 40(9): 2971-2979. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||