Chinese Journal of Organic Chemistry ›› 2024, Vol. 44 ›› Issue (11): 3518-3525.DOI: 10.6023/cjoc202405018 Previous Articles Next Articles
ARTICLE
收稿日期:2024-05-14
发布日期:2024-05-30
基金资助:Received:2024-05-14
Published:2024-05-30
Contact:
*E-mail:Supported by:Share
Qinghao Sun, Xiaoguang Bao. Computational Insights into the Mechanism of the Mo-Catalyzed Deoxygenative Coupling of Aromatic Aldehydes[J]. Chinese Journal of Organic Chemistry, 2024, 44(11): 3518-3525.
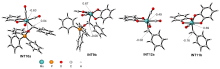
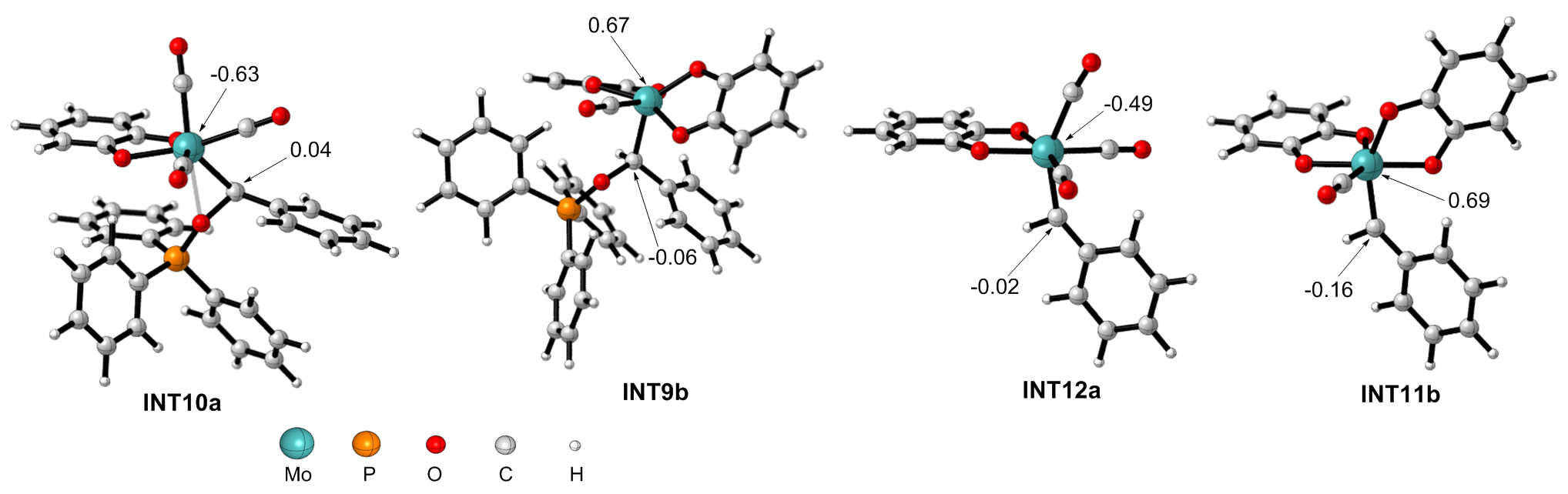
| [1] |
(a) Skell P. S.; Woodworth R. C. J. Am. Chem. Soc. 1956, 78, 4496.
pmid: 37123600 |
|
(b) Doyle M. P. Chem. Rev. 1986, 86, 919.
pmid: 37123600 |
|
|
(c) Xia Y.; Qiu D.; Wang J. Chem. Rev. 2017, 117, 13810.
pmid: 37123600 |
|
|
(d) Bergstrom B. D.; Nickerson L. A.; Shaw J. T.; Souza L. W. Angew. Chem., Int. Ed. 2021, 60, 6864.
pmid: 37123600 |
|
|
(e) Epping R. F. J.; Vesseur D.; Zhou M.; De Bruin B. ACS Catal. 2023, 13, 5428.
doi: 10.1021/acscatal.3c00591 pmid: 37123600 |
|
| [2] |
For metal-carbene involving C—H insertions, see: (a) Choi M. K. W.; Yu W. Y.; Che C. M. Org. Lett. 2005, 7, 1081.
|
|
(b) Doyle M. P.; Duffy R.; Ratnikov M.; Zhou L. Chem. Rev. 2010, 110, 704.
|
|
|
(c) Lo V. K. Y.; Guo Z.; Choi M. K. W.; Yu W. Y.; Huang J. S.; Che C.-M. J. Am. Chem. Soc. 2012, 134, 7588.
|
|
|
(d) Shen H.; Xiao X.; Haj M. K.; Willoughby P. H.; Hoye T. R. J. Am. Chem. Soc. 2018, 140, 15616.
|
|
|
(e) He Y.; Huang Z.; Wu K.; Ma J.; Zhou Y.-G.; Yu Z. Chem. Soc. Rev. 2022, 51, 2759.
|
|
| [3] |
For metal-carbene involving X—H insertions, see: (a) Gillingham D.; Fei N. Chem. Soc. Rev. 2013, 42, 4918.
doi: 10.1039/c3cs35496b pmid: 35748338 |
|
(b) Yang Z.; Stivanin M. L.; Jurberg I. D.; Koenigs R. M. Chem. Soc. Rev. 2020, 49, 6833.
pmid: 35748338 |
|
|
(c) Chen P.; Nan J.; Hu Y.; Kang Y.; Wang B.; Ma Y.; Szostak M. Chem. Sci. 2021, 12, 803.
pmid: 35748338 |
|
|
(d) Roose T. R.; Verdoorn D. S.; Mampuys P.; Ruijter E.; Maes B. U. W.; Orru R. V. A. Chem. Soc. Rev. 2022, 51, 5842.
doi: 10.1039/d1cs00305d pmid: 35748338 |
|
| [4] |
For metal-carbene involving olefin metathesis, see: (a) Schrock R. R.; Hoveyda A. H. Angew. Chem., Int. Ed. 2003, 42, 4592.
pmid: 29714397 |
|
(b) Grela K. Olefin Metathesis—Theory and Practice, Wiley, Hoboken, NJ, 2014.
pmid: 29714397 |
|
|
(c) Ogba O. M.; Warner N. C.; O'Leary D. J.; Grubbs R. H. Chem. Soc. Rev. 2018, 47, 4510.
doi: 10.1039/c8cs00027a pmid: 29714397 |
|
|
(d) Goudreault A. Y.; Walden D. M.; Nascimento D. L.; Botti A. G.; Steinmann S. N.; Michel C.; Fogg D. E. ACS Catal. 2020, 10, 3838.
pmid: 29714397 |
|
| [5] |
For metal-carbene involving cyclization, see: (a) Dai X.-J.; Li C.-C.; Li C.-J. Chem. Soc. Rev. 2021, 50, 10733.
|
|
(b) Zhang Y.-H.; Shi B.-F.; Yu J.-Q. J. Am. Chem. Soc. 2009, 131, 5072.
|
|
| [6] |
Wang T.; Hashmi A. S. K. Chem. Rev. 2021, 121, 8948.
doi: 10.1021/acs.chemrev.0c00811 pmid: 33026800 |
| [7] |
(a) Feliciano A.; Vázquez J. L.; Benítez-Puebla L. J.; Velazco- Cabral I.; Cruz D.; Delgado F.; Vázquez M. A. Chem.-Eur. J. 2021, 27, 8233.
pmid: 15137791 |
|
(b) Barluenga J.; Santamaría J.; Tomás M. Chem. Rev. 2004, 104, 2259.
pmid: 15137791 |
|
|
(c) Schrock R. R. Chem. Rev. 2002, 102, 145.
pmid: 15137791 |
|
|
(d) Schrock R. R. Chem. Rev. 2009, 109, 3211.
pmid: 15137791 |
|
|
(e) Frenking G.; Solà M.; Vyboishchikov S. F. J. Organomet. Chem. 2005, 690, 6178.
pmid: 15137791 |
|
| [8] |
Harvey D.; Brown M. J. Am. Chem. Soc. 1990, 112, 7806.
|
| [9] |
Schrock R. R.; Murdzek J. S.; Bazan G. C.; Robbins J.; DiMare M.; O'Regan M. J. Am. Chem. Soc. 1990, 112, 3875.
|
| [10] |
(a) Asako S.; Ishihara S.; Hirata K.; Takai K. J. Am. Chem. Soc. 2019, 141, 9832.
doi: 10.1021/jacs.9b05428 pmid: 31184481 |
|
(b) Banerjee S.; Kobayashi T.; Takai K.; Asako S.; Ilies L. Org. Lett. 2022, 24, 7242.
pmid: 31184481 |
|
|
(c) Asako S.; Kobayashi T.; Ishihara S.; Takai K. Asian J. Org. Chem. 2021, 10, 753.
doi: 10.1002/ajoc.202100038 pmid: 31184481 |
|
| [11] |
(a) Cao L.-Y.; Luo J.-N.; Yao J.-S.; Wang D.-K.; Dong Y.-Q.; Zheng C.; Zhuo C.-X. Angew. Chem., Int. Ed. 2021, 60, 15254.
|
|
(b) Dong Y.-Q.; Wang K.; Zhuo C.-X. ACS Catal. 2022, 12, 11428.
|
|
|
(c) Cao L.-Y.; Wang J.-L.; Wang K.; Wu J.-B.; Wang D.-K.; Peng J.-M.; Bai J.; Zhuo C.-X. J. Am. Chem. Soc. 2023, 145, 2765.
|
|
|
(d) Yu Y.-Z.; Bai J.; Peng J.-M.; Yao J.-S.; Zhuo C.-X. J. Am. Chem. Soc. 2023, 145, 8781.
|
|
|
(e) Dong Y.-Q.; Shi X.-N.; Cao L.-Y.; Bai J.; Zhuo C.-X. Org. Chem. Front. 2023, 10, 3544.
|
|
| [12] |
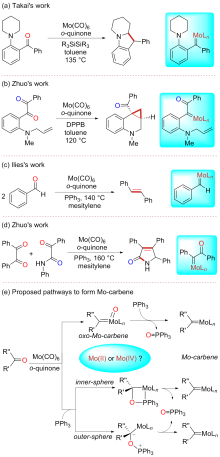
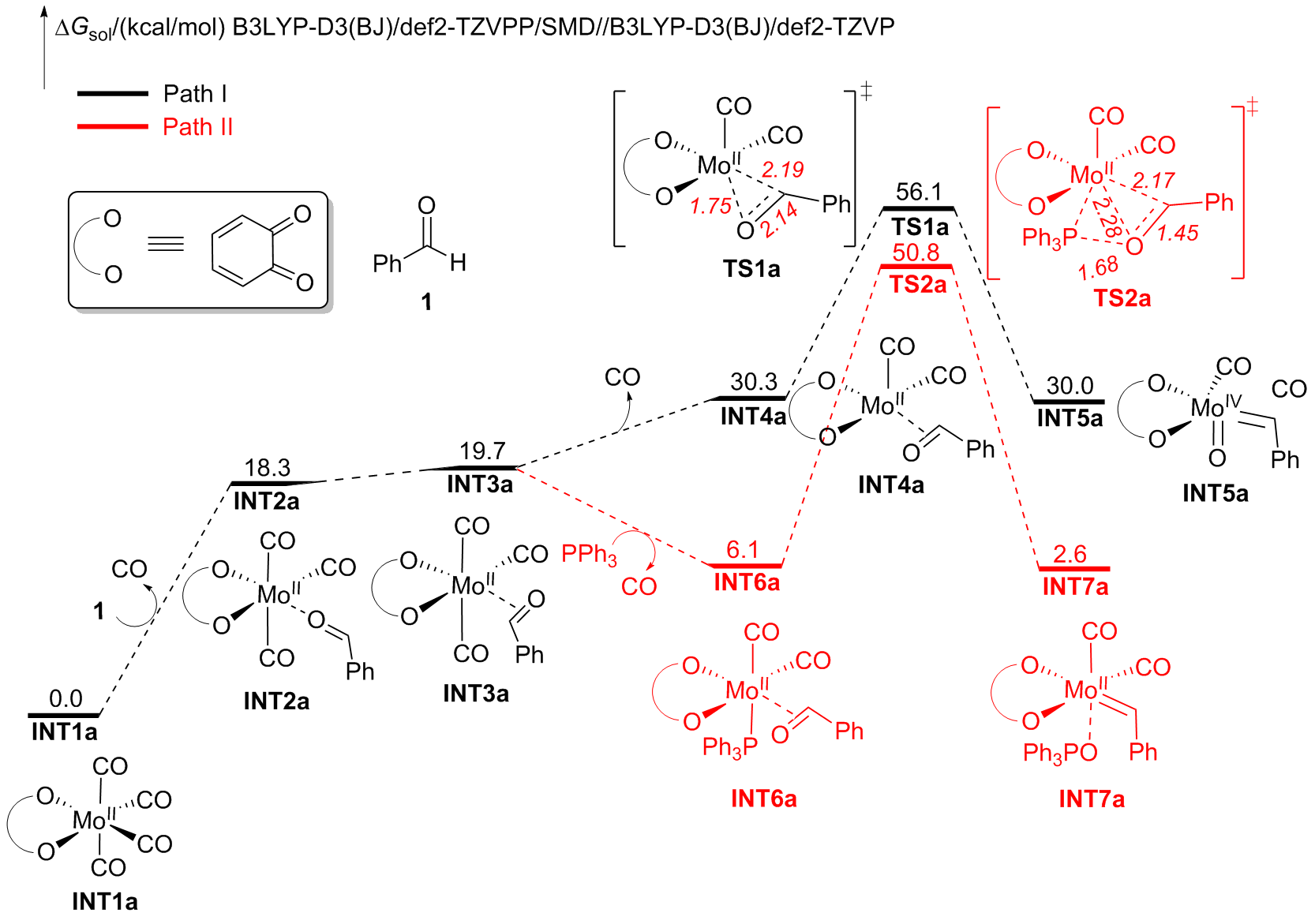
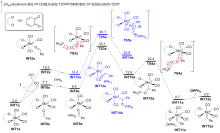
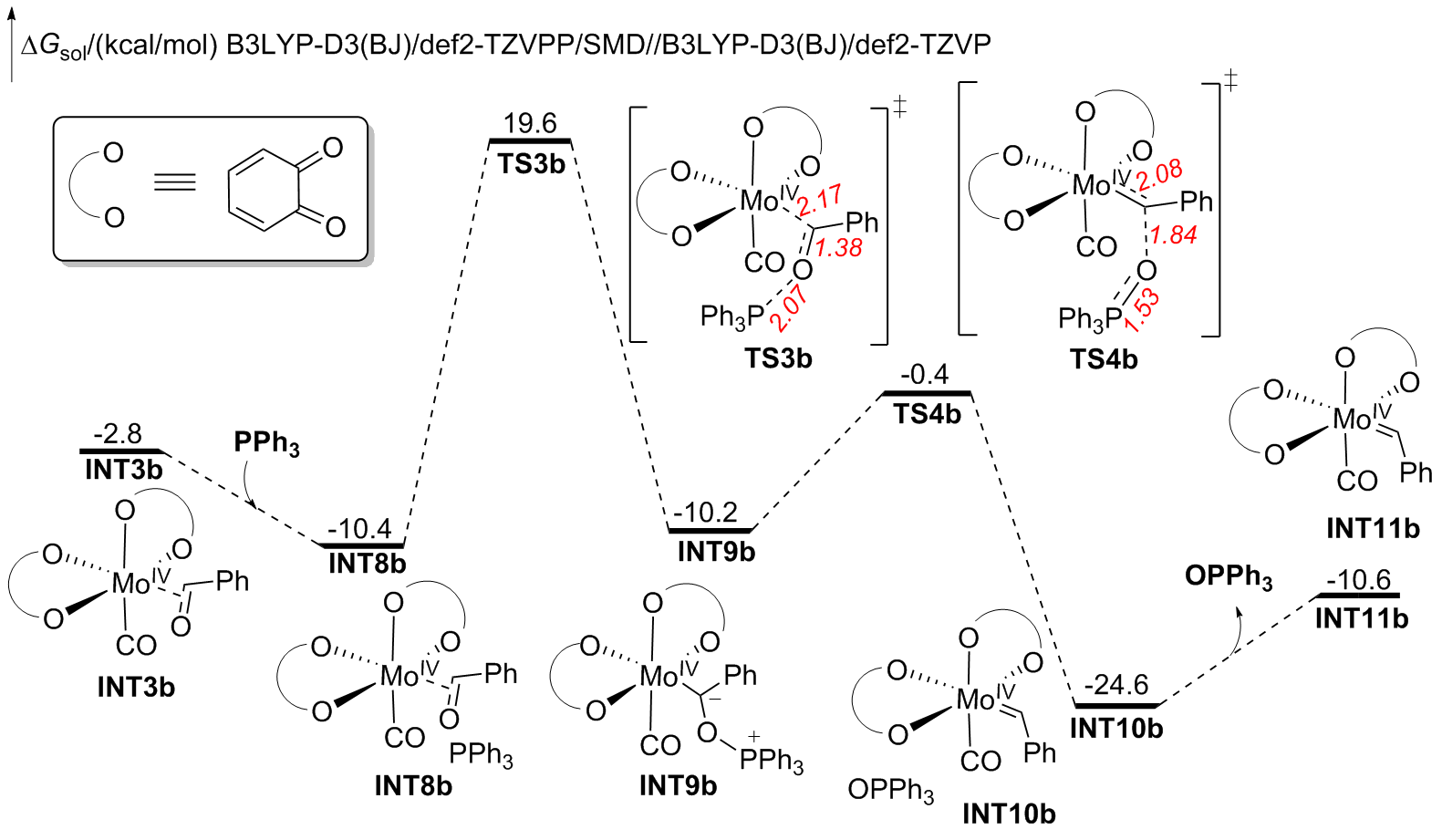
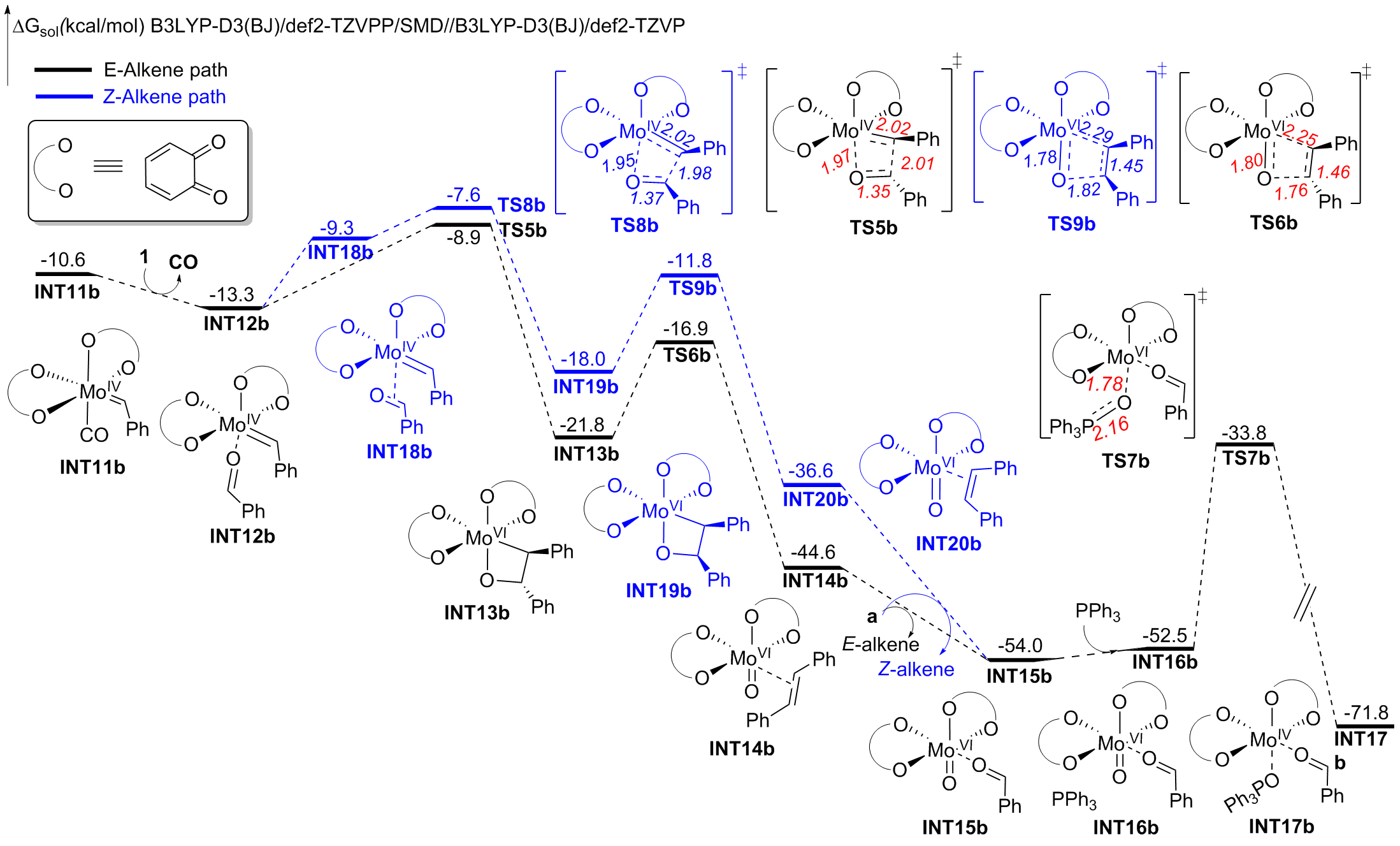
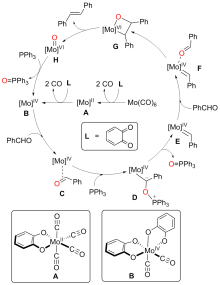
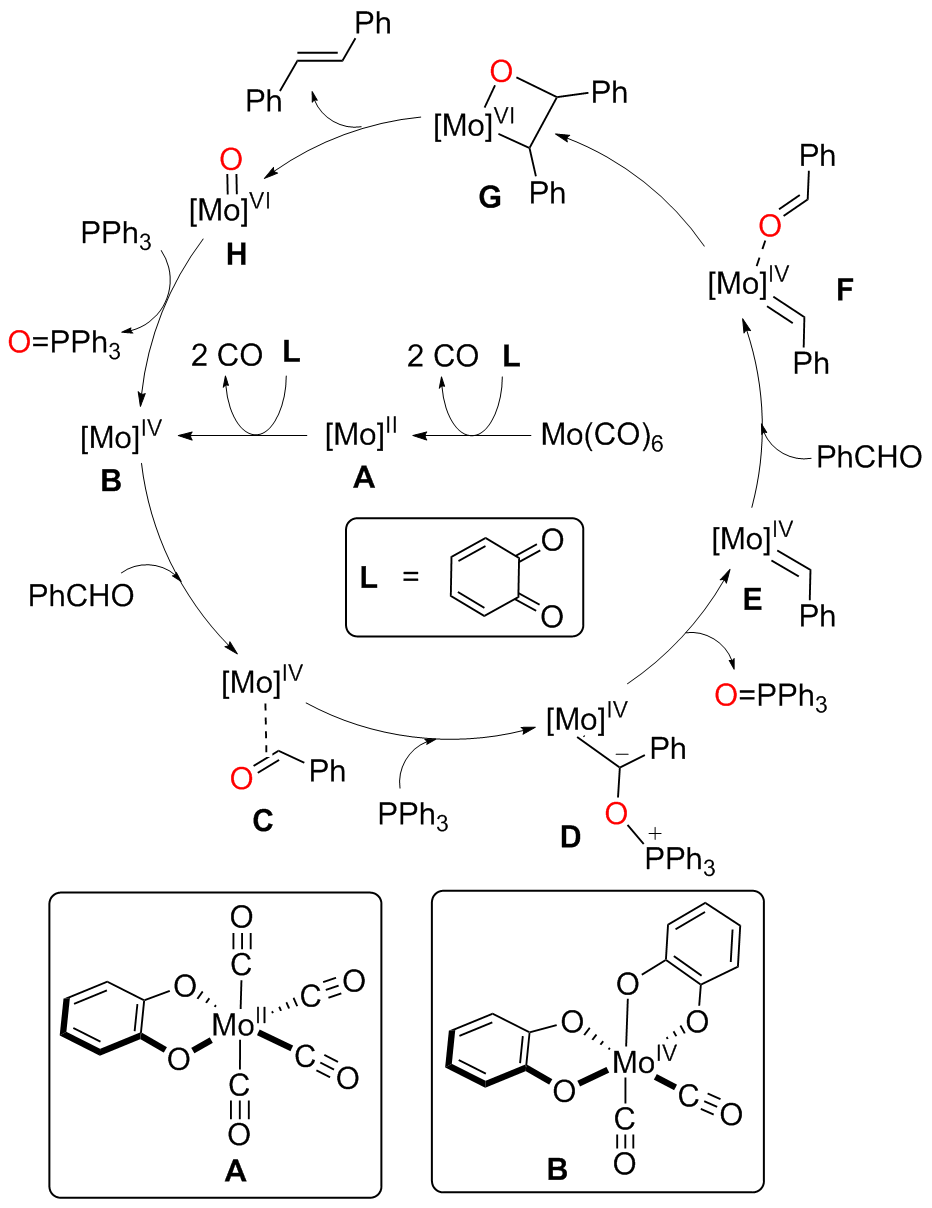
A direct C=O bond cleavage of 1 via path I on equatorial plane to give an oxo-Mo-carbene can also be ruled out due to the even higher activation barrier (Figure S1).
|
| [13] |
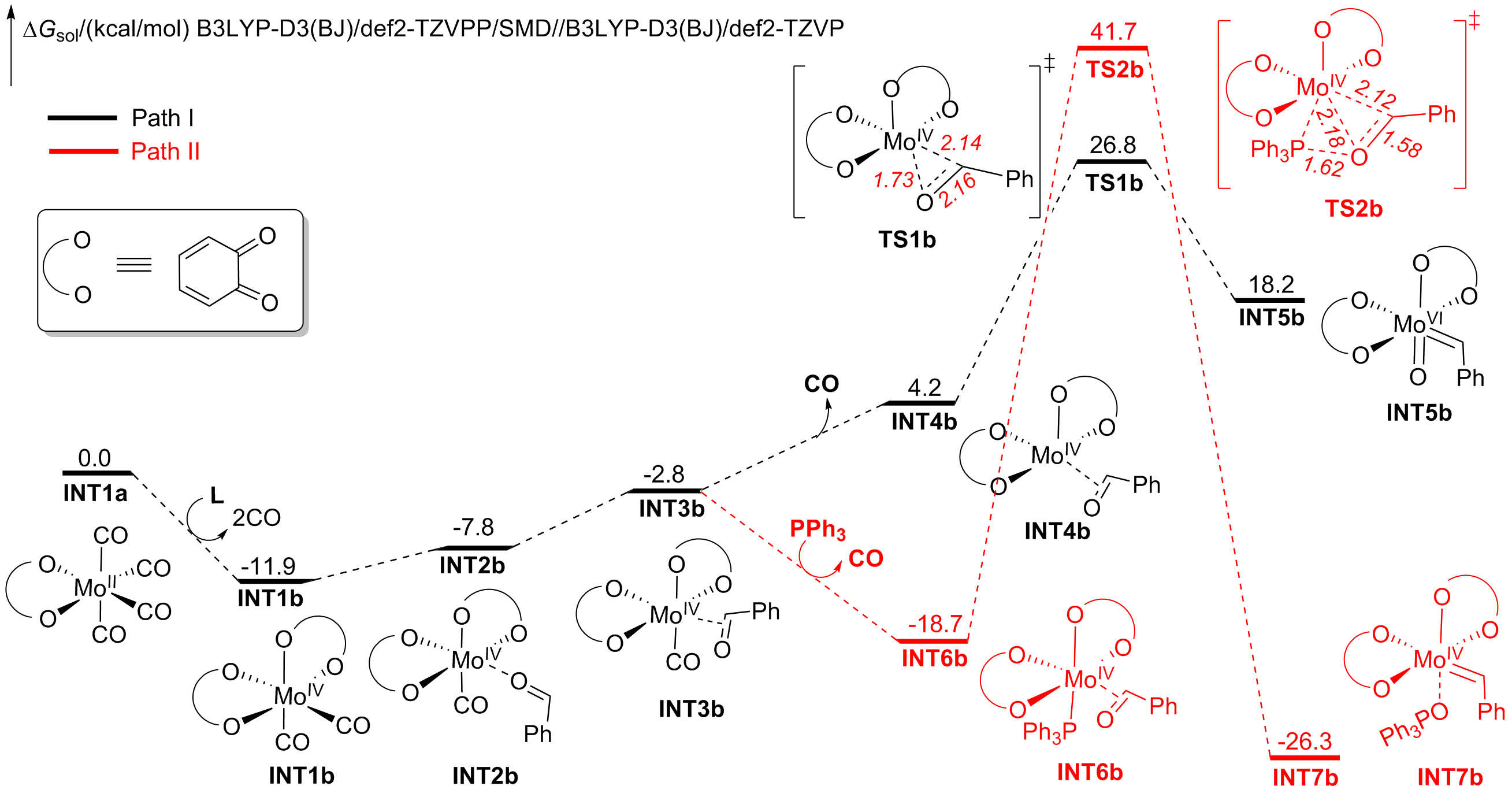
A nucleophilic attack via the oxygen atom of the carbonyl group to the carbene moiety of INT12b/INT12b' via TS5b'/TS5b'' could be excluded due to the much higher activation barrier (Figure S3). The Schrock-type carbene character with the negatively charged carbene carbon in INT12b is responsible for the chemo-selectivity.
|
| [14] |
The PPh3 additive assisted deoxygenation of the oxo-Mo-carbene in a reductive elimination style via TS7b' can be excluded due to the much higher activation barrier (Figure S5).
|
| [15] |
(a) Becke A. D. J. Chem. Phys. 1993, 98, 5648.
pmid: 9944570 |
|
(b) Lee C.; Yang W.; Parr R. G. Phys. Rev. B: Condens. Matter Mater. Phys. 1988, 37, 785.
doi: 10.1103/physrevb.37.785 pmid: 9944570 |
|
|
(c) Grimme S.; Antony J.; Ehrlich S.; Krieg H. J. Chem. Phys. 2010, 132, 154104.
pmid: 9944570 |
|
| [16] |
(a) Schaefer A.; Horn H.; Ahlrichs R. J. Chem. Phys. 1992, 97, 2571.
|
|
(b) Schaefer A.; Huber C.; Ahlrichs R. J. Chem. Phys. 1994, 100, 5829.
|
|
|
(c) Weigend F.; Ahlrichs R. Phys. Chem. Chem. Phys. 2005, 7, 3297.
|
|
| [17] |
(a) Fukui K. J. Phys. Chem. 1970, 74, 4161.
|
|
(b) Fukui K. Acc. Chem. Res. 1981, 14, 363.
|
|
| [18] |
Marenich A. V.; Cramer C. J.; Truhlar D. G. J. Phys. Chem. B 2009, 113, 6378.
|
| [19] |
Martin R. L.; Hay P. J.; Pratt L. R. J. Phys. Chem. A 1998, 102, 3565.
|
| [20] |
(a) Li H.; Jiang J.; Lu G.; Huang F.; Wang Z.-X. Organometallics 2011, 30, 3131.
|
|
(b) Li H.; Wen M.; Wang Z.-X. Inorg. Chem. 2012, 51, 5716.
|
|
|
(c) Wen M.; Huang F.; Lu G.; Wang Z.-X. Inorg. Chem. 2013, 52, 12098.
|
|
|
(d) Qu S.; Dang Y.; Song C.; Wen M.; Huang K.-W.; Wang Z.-X. J. Am. Chem. Soc. 2014, 136, 4974.
|
|
|
(e) Yu J.-L.; Zhang S.-Q.; Hong X. J. Am. Chem. Soc. 2017, 139, 7224.
|
|
| [21] |
Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; Bloino J.; Zheng G.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Montgomery J. A.; Peralta J. E.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Staroverov V. N.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam N. J.; Klene M.; Knox J. E.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J. J.; Dapprich S.; Daniels A. D.; Farkas O.; Foresman J. B.; Ortiz J. V.; Cioslowski J.; Fox D. J. Gaussian 09, revision C.01, Gaussian, Inc., Wallingford, CT, 2010.
|
| [22] |
Legault C. Y. CYLview 1.0b, CYLview 1.0b, Université de Sherbrooke, Sherbrooke, Quebec, Canada, 2009, http://www.Cylview.org
|
| [23] |
Johnson E. R.; Keinan S.; Mori-Sánchez P.; Contreras-Garcia J.; Cohen A. J.; Yang W. J. Am. Chem. Soc. 2010, 132, 6498.
doi: 10.1021/ja100936w pmid: 20394428 |
| [24] |
Lu T.; Chen F. J. Comput. Chem. 2012, 33, 580.
|
| [25] |
Humphrey W.; Dalke A.; Schulten K. J. Mol. Graph. 1996, 14, 33.
doi: 10.1016/0263-7855(96)00018-5 pmid: 8744570 |
| [1] | Jia-Xi Jiang, Quan-Zhong Liu. Non-Metallic Carbene Pathway Transformations of Vinyl Diazo Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(9): 2640-2657. |
| [2] | Ming Zhao, Rui Yan, Hu Chen. N-Heterocyclic Carbene Catalyzed the Umpolung of Aldehyde Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(7): 2204-2215. |
| [3] | Xiaodong Liu, Shiliang Shi. ANIPE-Ligand-Enabled Copper-Catalyzed Asymmetric Carboboronation of Allenes with Imines and Diborons [J]. Chinese Journal of Organic Chemistry, 2024, 44(6): 1884-1896. |
| [4] | Chao Sun, Quan Zhou, Chuanying Li, Lei Wang. Syntheses and Structural Characterizations of Benzoxazolyl N-Heterocyclic Carbene-Palladium Complexes and Their Applications [J]. Chinese Journal of Organic Chemistry, 2024, 44(6): 1957-1966. |
| [5] | Yan Liu, Xiaomei Wang, Lin He, Shiwu Li, Zhifei Zhao. N-Heterocyclic Carbene (NHC)-Catalyzed [3+2] Cycloaddition to Highly Diastereoselective Synthesis of Spirooxindole Dihydrofuran Fused Pyrazolone Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(4): 1301-1310. |
| [6] | Dengpeng Xia, Jinyun Luo, Lin He, Zhihua Cai, Guangfen Du. N-Heterocyclic Carbene-Catalyzed Synthesis of Pentafluorophenyl Sulfides [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 622-630. |
| [7] | Shuang Yang, Xinqiang Fang. Kinetic Resolutions Enabled by N-Heterocyclic Carbene Catalysis: An Update [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 448-480. |
| [8] | Xuewei Chen, Fangcai Yu, Chuanhong Tian. 1,1'-Methylenediimidazolium-Based Multiple Hydrogen-Bond Donor Catalysts Facilitate the Cycloaddition of CO2 with Epoxides under Atmospheric Pressure [J]. Chinese Journal of Organic Chemistry, 2024, 44(10): 3198-3205. |
| [9] | Huanqing Li, Zhaohua Chen, Zujia Chen, Qiwen Qiu, Youcai Zhang, Sihong Chen, Zhaoyang Wang. Research Progress in Mercury Ion Fluorescence Probes Based on Organic Small Molecules [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3067-3077. |
| [10] | Yuanlin Cai, Ya Lü, Guihua Nie, Zhichao Jin, Yonggui Chi. Recent Advances in the Synthesis of Cyanides Enabled by N-Heterocyclic Carbene Catalysis [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3135-3145. |
| [11] | Xiaoping Xu, Yifei Zhang, Xiaoyu Mo, Jun Jiang. Rh-Catalyzed C—H Functionalization Reaction between 3-Diazoindolin-2-imines and Pyrazolones for the Construction of 3-Pyrazolyl Indoles [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2519-2527. |
| [12] | Liangru Yang, Mengli Guo, Jinwei Yuan, Jiamei Wangx, Yuting Xia, Yongmei Xiao, Pu Mao. Research Progress on Pincer N-Heterocyclic Carbene Metal Complexes [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2002-2025. |
| [13] | Zhihao Chen, Qi Fan, Biaolin Yin, Qingjiang Li, Honggen Wang. Progress in the Syntheses of α-Boryl Carbonyl Compounds [J]. Chinese Journal of Organic Chemistry, 2023, 43(5): 1706-1712. |
| [14] | Cunjing Miao, Jiaqi Yao. Recent Advances in the Transformation Reactions of Aromatic Nitriles via C—CN Bond Cleavage [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1341-1364. |
| [15] | Xinyu Zhang, Huihui Geng, Shilei Zhang, Wei Wang, Xiaobei Chen. A Method for the Synthesis of Deuterated Benzoins Catalyzed by N-Heterocyclic Carbene [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1510-1516. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
