-
-
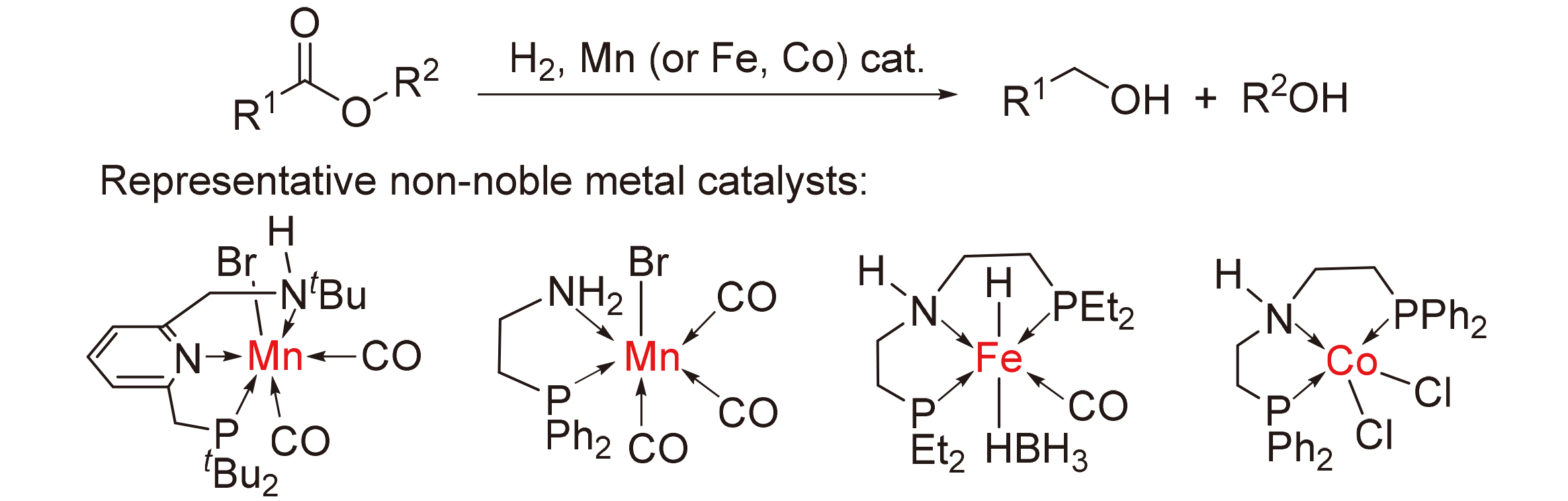
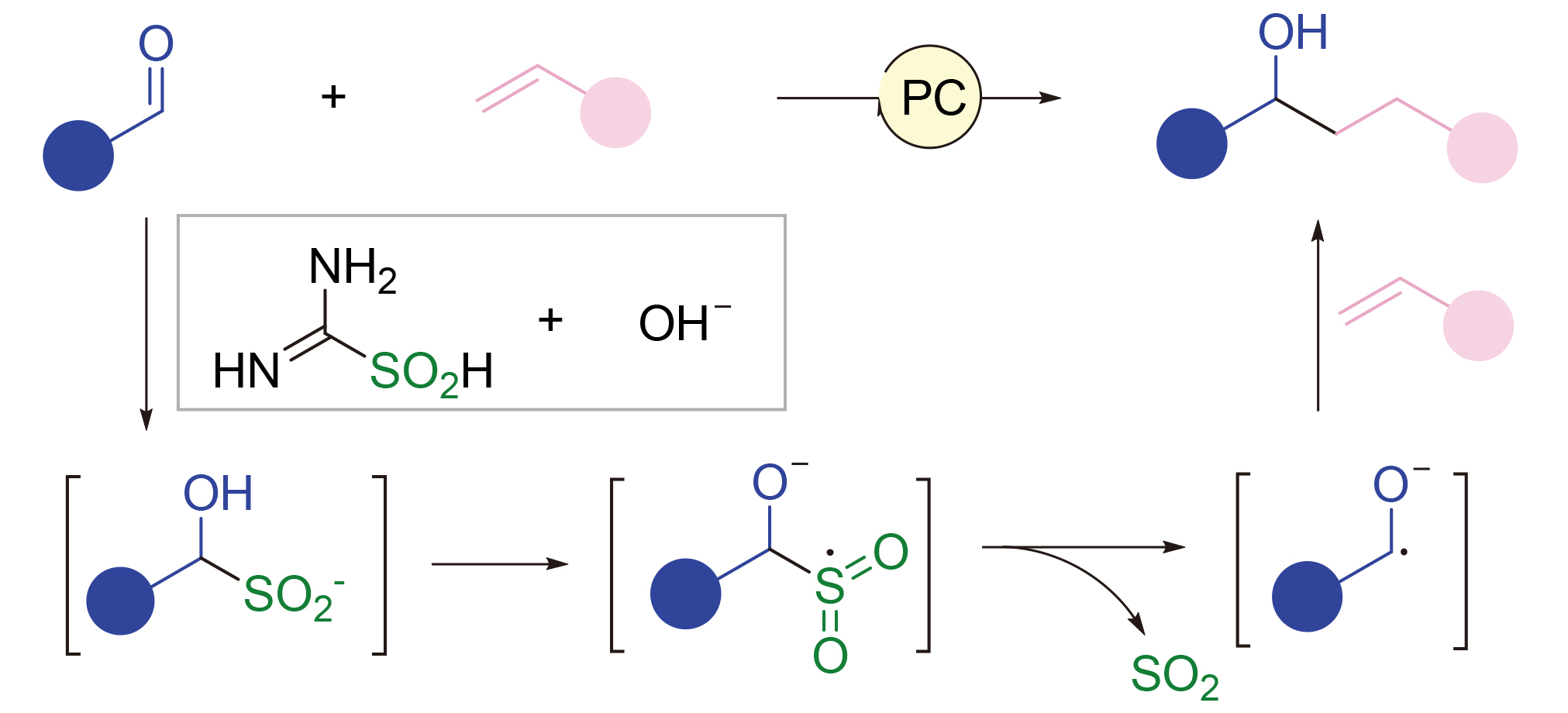
 About the Cover:Selective functionalization of hydrocarbon chains is an important scientific problem in synthetic chemistry, and direct functionaliza-tion of easily available alkanes or alkenes is an ideal method for their efficient and high value-added conversion. A comprehensive overview of the recent developments in se-lective multi-site functionalization of hydro-carbon chains is provided by Wang, Liu, Yang, Wu and Zhang on page 3273.
About the Cover:Selective functionalization of hydrocarbon chains is an important scientific problem in synthetic chemistry, and direct functionaliza-tion of easily available alkanes or alkenes is an ideal method for their efficient and high value-added conversion. A comprehensive overview of the recent developments in se-lective multi-site functionalization of hydro-carbon chains is provided by Wang, Liu, Yang, Wu and Zhang on page 3273. -
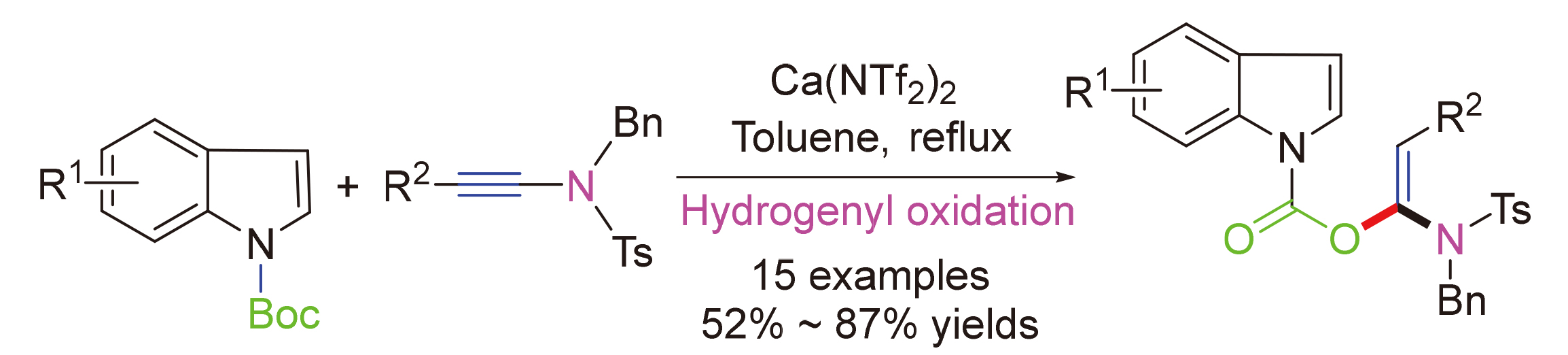
 About the Cover:Amidoenol structural fragments are widely found in bioactive natural products and pharmaceutical compounds. The addition reaction of tert-butyl 1-indole- carboxylate and ynamides, catalyzed by Ca(NTf2)2, which enables the construction of key indole amidoenol fragments, is reported by Zhang, Song, Li, Zheng and Wei on page 3490. The method demonstrates stable yields, high regioselectivities, and well substrate suitabil-ities.
About the Cover:Amidoenol structural fragments are widely found in bioactive natural products and pharmaceutical compounds. The addition reaction of tert-butyl 1-indole- carboxylate and ynamides, catalyzed by Ca(NTf2)2, which enables the construction of key indole amidoenol fragments, is reported by Zhang, Song, Li, Zheng and Wei on page 3490. The method demonstrates stable yields, high regioselectivities, and well substrate suitabil-ities. -
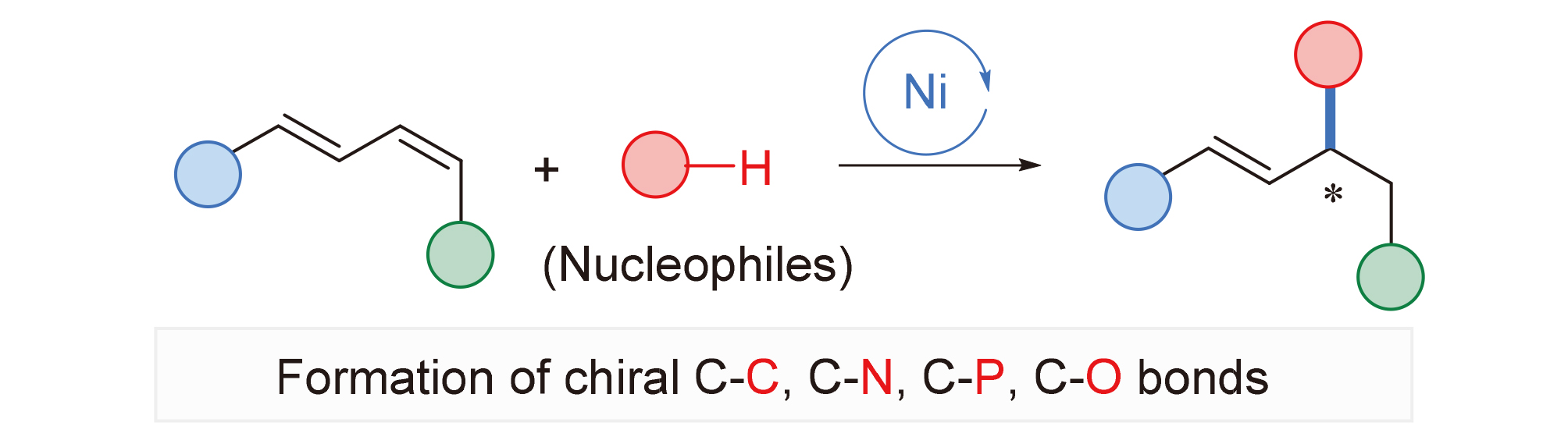
 About the Cover:The recent progress of nickel-catalyzed asymmetric hydrofunctionalization of 1,3-dienes with different nucleophiles is reviewed by Long, Liu, Zhang, Zhu and Gu on page 3309. The substrate scope, limita-tions and mechanism characteristics of asymmetric hydrofunctionalization are dis-cussed. According to the different kinds of nucleophiles, it mainly includes asymmetric hydrocarbonation, asymmetric hydroamina-tion, asymmetric hydrophosphinylation and asymmetric hydroalkoxylation.
About the Cover:The recent progress of nickel-catalyzed asymmetric hydrofunctionalization of 1,3-dienes with different nucleophiles is reviewed by Long, Liu, Zhang, Zhu and Gu on page 3309. The substrate scope, limita-tions and mechanism characteristics of asymmetric hydrofunctionalization are dis-cussed. According to the different kinds of nucleophiles, it mainly includes asymmetric hydrocarbonation, asymmetric hydroamina-tion, asymmetric hydrophosphinylation and asymmetric hydroalkoxylation.
-
-
Current Issue
REVIEW
ARTICLE
NOTE
HIGHLIGHT