Chinese Journal of Organic Chemistry ›› 2025, Vol. 45 ›› Issue (10): 3903-3911.DOI: 10.6023/cjoc202412011 Previous Articles Next Articles
ARTICLES
刘菲a, 徐鑫明a,*( ), 王薪a, 刘春阳b,*(
), 王薪a, 刘春阳b,*( ), 陈雪波b, 孙凯a,*(
), 陈雪波b, 孙凯a,*( )
)
收稿日期:2024-12-15
修回日期:2025-01-05
发布日期:2025-02-07
基金资助:
Fei Liua, Xinming Xua,*( ), Xin Wanga, Chunyang Liub,*(
), Xin Wanga, Chunyang Liub,*( ), Xuebo Chenb, Kai Suna,*(
), Xuebo Chenb, Kai Suna,*( )
)
Received:2024-12-15
Revised:2025-01-05
Published:2025-02-07
Contact:
Supported by:Share
Fei Liu, Xinming Xu, Xin Wang, Chunyang Liu, Xuebo Chen, Kai Sun. Direct Nitration of Enamide to β-Acylamino Nitroalkenes under Metal-Free Conditions[J]. Chinese Journal of Organic Chemistry, 2025, 45(10): 3903-3911.
| Entry | Oxidant | Solventb | T/℃ | t/h | Yieldc/% | |
|---|---|---|---|---|---|---|
| 1 | K2S2O8 | 1,4-Dioxane | 90 | 13 | 63 | |
| 2 | TBHP | 1,4-Dioxane | 90 | 13 | 60 | |
| 3 | DTBP | 1,4-Dioxane | 90 | 13 | 70 | |
| 4 | DCP | 1,4-Dioxane | 90 | 13 | 56 | |
| 5 | TEMPO | 1,4-Dioxane | 90 | 13 | 74 | |
| 6 | BPO | 1,4-Dioxane | 90 | 13 | 58 | |
| 7 | TEMPO | THF | 90 | 14 | 72 | |
| 8 | TEMPO | DCE | 90 | 12 | 62 | |
| 9 | TEMPO | Toluene | 90 | 14 | 61 | |
| 10 | TEMPO | CH3CN | 90 | 8 | 88 | |
| 11 | TEMPO | CH3CN | 80 | 9 | 73 | |
| 12 | TEMPO | CH3CN | 100 | 8 | 78 | |
| 13 | TEMPO | CH3CN | 110 | 8 | 75 | |
| 14d | TEMPO | CH3CN | 90 | 12 | 66 | |
| 15e | TEMPO | CH3CN | 90 | 12 | 77 | |
| 16f | TEMPO | CH3CN | 90 | 16 | 64 | |
| 17g | TEMPO | CH3CN | 90 | 16 | 41 | |
| 18h | TEMPO | CH3CN | 90 | 16 | 8 | |
| Entry | Oxidant | Solventb | T/℃ | t/h | Yieldc/% | |
|---|---|---|---|---|---|---|
| 1 | K2S2O8 | 1,4-Dioxane | 90 | 13 | 63 | |
| 2 | TBHP | 1,4-Dioxane | 90 | 13 | 60 | |
| 3 | DTBP | 1,4-Dioxane | 90 | 13 | 70 | |
| 4 | DCP | 1,4-Dioxane | 90 | 13 | 56 | |
| 5 | TEMPO | 1,4-Dioxane | 90 | 13 | 74 | |
| 6 | BPO | 1,4-Dioxane | 90 | 13 | 58 | |
| 7 | TEMPO | THF | 90 | 14 | 72 | |
| 8 | TEMPO | DCE | 90 | 12 | 62 | |
| 9 | TEMPO | Toluene | 90 | 14 | 61 | |
| 10 | TEMPO | CH3CN | 90 | 8 | 88 | |
| 11 | TEMPO | CH3CN | 80 | 9 | 73 | |
| 12 | TEMPO | CH3CN | 100 | 8 | 78 | |
| 13 | TEMPO | CH3CN | 110 | 8 | 75 | |
| 14d | TEMPO | CH3CN | 90 | 12 | 66 | |
| 15e | TEMPO | CH3CN | 90 | 12 | 77 | |
| 16f | TEMPO | CH3CN | 90 | 16 | 64 | |
| 17g | TEMPO | CH3CN | 90 | 16 | 41 | |
| 18h | TEMPO | CH3CN | 90 | 16 | 8 | |


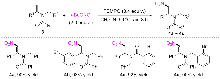

| [1] |
|
| [2] |
doi: 10.1126/science.1158140 pmid: 18621671 |
| [3] |
|
| [4] |
|
| [5] |
doi: 10.1021/ol303314x pmid: 23268775 |
| [6] |
|
| [7] |
pmid: 11950357 |
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
doi: 10.1007/s12013-013-9709-2 pmid: 23794010 |
| [14] |
|
| [15] |
doi: 10.1021/jm1004286 pmid: 21105711 |
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
doi: 10.1021/acs.orglett.8b00058 pmid: 29412678 |
| [20] |
pmid: 10814045 |
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
doi: 10.1021/acs.orglett.6b00175 pmid: 26886569 |
| [31] |
|
| [32] |
|
| [33] |
doi: 10.1021/acs.orglett.6b01255 pmid: 27206072 |
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
doi: 10.1021/ja512212x pmid: 25545361 |
| [38] |
doi: 10.1021/ol301801r pmid: 22891899 |
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
doi: 10.1039/c4ob01970a pmid: 25354883 |
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [1] | Yiwen Lü, Haihua Liao, Wenjun Tang. Synthesis of a Sitagliptin Intermediate via Ruthenium-Catalyzed Asymmetric Hydrogenation of Enamine Ester [J]. Chinese Journal of Organic Chemistry, 2024, 44(12): 3720-3726. |
| [2] | Zhaoxin Wei, Renjie Wang, Yonghong Zhang, Bin Wang, Yu Xia, Weiwei Jin, Chenjiang Liu. Electrochemical Synthesis of N-Acyl/Sulfonylsulfenamides Using Potassium Iodide as Mediator [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3730-3739. |
| [3] | Weiwei Wang, Yihao Li, Xinlei Liu, Yu Zhao, Mingan Wang. Synthesis and Fungicidal Activity of Novel 3,7-Dimethylocta-2,6-dienamides and 3,7-Dimethyl-6,7-dihydroxyoct-2-enamides [J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3717-3725. |
| [4] | Muxue He, Shiyan Cheng, Yongzhou Pan, Haitao Tang, Yingming Pan. Electrochemically Mediated S—N Bond Formation: Synthesis of Sulfenamides [J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2354-2360. |
| [5] | Lingling Zhang, Zhi Wang, Jian Wu, Xiaoqing Li. Synthesis of Nitro-Functionalized Isoquinolinediones via NaNO2/Na2S2O8-Mediated Arylnitration of Alkenes [J]. Chinese Journal of Organic Chemistry, 2021, 41(4): 1734-1738. |
| [6] | Yinjun Huang, Jinshan Li, Shen Li, Junan Ma. Cobalt-Catalyzed Aerobic Oxidative Dearomatization of 2-Aryl Indoles and in situ [3+2] Annulation with Enamides [J]. Chinese Journal of Organic Chemistry, 2021, 41(10): 4028-4038. |
| [7] | Bing Mu, Junliang Wu, Guang'an Zhang. Alternative Approach for the Synthesis of Nitroaromatic Olefins via Dehydrogenative Nitration of Easily Available Arylethanes [J]. Chinese Journal of Organic Chemistry, 2021, 41(1): 250-257. |
| [8] | Zhang Zhu, Li Jiazhu, Wang Xinyue, Ma Jihua, Wang Xu, Wang Jinjun. Nitration (Nitroalkylation) of Pheophorbide and Synthesis of Chlorophyllous Chlorin Derivatives [J]. Chinese Journal of Organic Chemistry, 2020, 40(9): 2895-2903. |
| [9] | Zhou Xiaoqiang, Yan Hao, Wang Qiuya. N-Iodo-succininide (NIS)/K2S2O8 Initiated Self-Coupling of Enamides to Nitrogen-Containing Quaternary Carbon Centers [J]. Chinese Journal of Organic Chemistry, 2020, 40(7): 2142-2147. |
| [10] | Wang Qiushi, Xie Jianhua, Zhou Qilin. Ruthenium Catalyzed Highly Chemo-and Regio-selective Codimerization of N-Acetyl α-Arylethenamines with Vinylarenes [J]. Chin. J. Org. Chem., 2019, 39(8): 2264-2269. |
| [11] | Xia Liwen, Zhao Qing, Ba Mengyu, Hu chaoping, Sun Moran, Yang Hua. A Synthetic Route to Access Allyl-methyl-N-pantothenamide via [2,3]-Wittig Rearrangement [J]. Chin. J. Org. Chem., 2019, 39(7): 2035-2041. |
| [12] | Wang Yunlong, Zhang Linbao, Niu Junlong, Song Maoping. Copper-Promoted Direct Nitration of Arenes Assisted by an N,O-Bidentate Directing System [J]. Chin. J. Org. Chem., 2019, 39(6): 1761-1766. |
| [13] | Feng Yige, Ye Zhegao, Hao Shiyou. Hydrogen Bond Driven Beckmann Rearrangement of Diphenyl-ketoxime [J]. Chin. J. Org. Chem., 2019, 39(4): 1122-1128. |
| [14] | Cheng Huicheng, Lin Jinlong, Zhang Yaofeng, Chen Bing, Wang Min, Cheng Lihua, Ma Jiaoli. Recent Advances in Transition-Metal-Catalyzed Directing Group Assisted Nitration of Inert C-H Bonds [J]. Chin. J. Org. Chem., 2019, 39(2): 318-327. |
| [15] | Geng Dianguo. Recent Advances on Transition-Metal-Catalyzed Allenamides Cyclization [J]. Chin. J. Org. Chem., 2019, 39(2): 301-317. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||