Chinese Journal of Organic Chemistry ›› 2019, Vol. 39 ›› Issue (10): 2829-2834.DOI: 10.6023/cjoc201903053 Previous Articles Next Articles
Special Issue: 荧光探针-生物传感合辑; 有机超分子化学合辑
ARTICLE
朱文博, 朱伟, 丁金东, 马小强, 姚红, 张有明, 林奇*( ), 魏太保*(
), 魏太保*( )
)
收稿日期:2019-03-24
修回日期:2019-05-13
发布日期:2019-06-06
通讯作者:
林奇,魏太保
E-mail:linqi2004@126.com;weitaibao@126.com
基金资助:
Zhu Wenbo, Zhu Wei, Ding Jindong, Ma Xiaoqiang, Yao Hong, Zhang Youming, Lin Qi*( ), Wei Taibao*(
), Wei Taibao*( )
)
Received:2019-03-24
Revised:2019-05-13
Published:2019-06-06
Contact:
Lin Qi,Wei Taibao
E-mail:linqi2004@126.com;weitaibao@126.com
Supported by:Share
Zhu Wenbo, Zhu Wei, Ding Jindong, Ma Xiaoqiang, Yao Hong, Zhang Youming, Lin Qi, Wei Taibao. A Novel Fluorescent Sensor Based on Aryl-furfural Functionalized Barbituric Acid for Recognition and Separation of Hg 2+/Fe 3+[J]. Chinese Journal of Organic Chemistry, 2019, 39(10): 2829-2834.
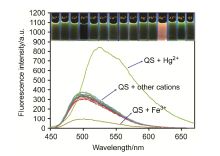
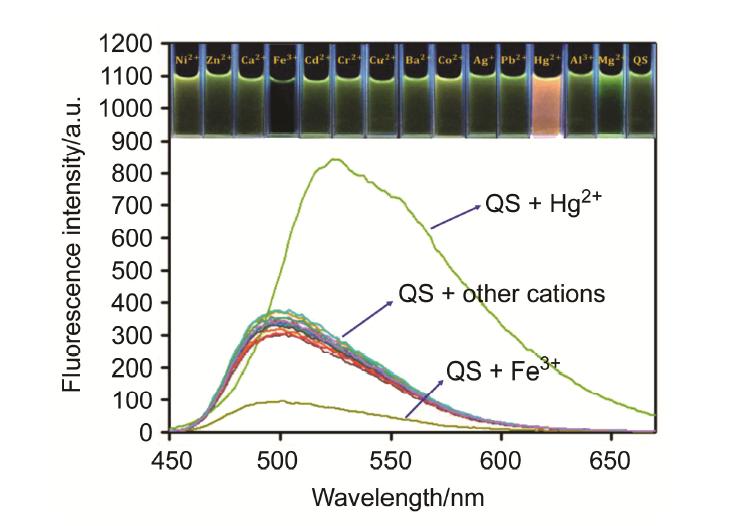
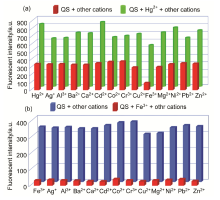
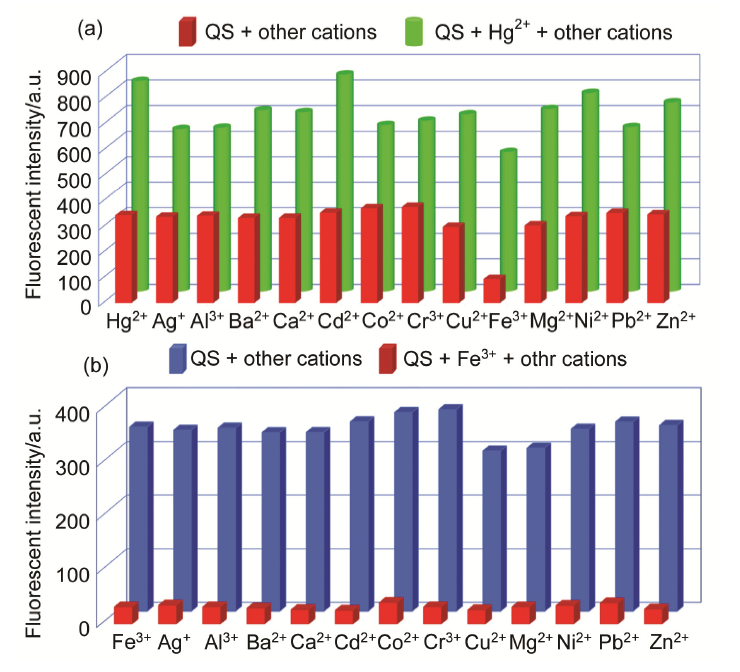
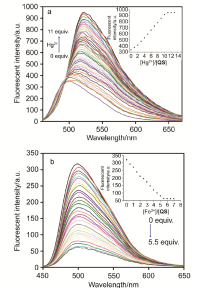

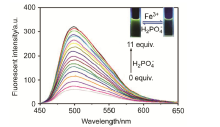
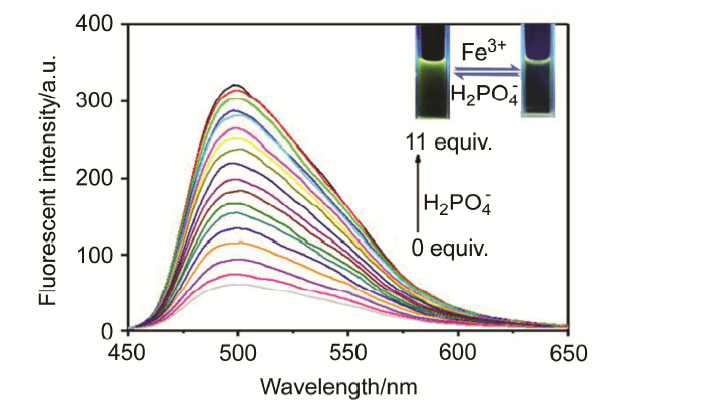
| [1] | Wang, J.; Li, Y.; Patel, N. G.; Zhang, G.; Zhou, D.; Pang, Y. Chem. Commun. 2014, 50, 12258. |
| [2] | Zhuang, P.; McBride, M. B.; Xia, H.; Li, N.; Li, Z. Sci. Total Environ. 2009, 407, 1551. |
| [3] | Yan, F.; Zou, Y.; Wang, M.; Mu, X.; Yang, N; Chen, L. . Sens. Actuators, B 2014, 192, 488. |
| [4] | Wu, X. F.; Ma, Q. J.; Wei, X. J.; Hou, Y. M.; Zhu, X. Sens. Actuators, B 2013, 183, 565. |
| [5] | Yue, Q.; Shen, T.; Wang, J.; Wang, L.; Xu, S.; Li, H.; Liu, J. Chem. Commun. 2013, 49, 1750. |
| [6] | Kim, H. N.; Ren, W. X.; Kim, J. S.; Yoon, J.S. Chem. Soc. Rev. 2012, 41, 3210. |
| [7] | López-García, I.; Rivas, R. E.; Hernández-Córdoba, M. Anal. Chim. Acta 2012, 743, 69. |
| [8] | Sun, Z.; Du, J.; Jing, C. J. Environ. Sci. 2016, 39, 134. |
| [9] | Yan, Z.; Hu, L.; You, J. Anal. Methods 2016, 8, 5738. |
| [10] | Wu, J.; Liu, W.; Ge, J.; Zhang, H.; Wang, P. Chem. Soc. Rev. 2011, 40, 3483. |
| [11] | Sahoo, S. K.; Sharma, D.; Bera, R. K.; Crisponic, G.; Callanm, J.F. Chem. Soc. Rev. 2012, 41, 7195. |
| [12] | Liu, Y.; Duan, W.; Song, W.; Liu, J.; Ren, C.; Wu, J.; Liu, D.; Chen, H. ACS Appl. Mater. Interfaces 2017, 9, 12663. |
| [13] | Rauchfus, T. B . Acc. Chem. Res. 2015, 48, 2107. |
| [14] |
Zhang, S.; Li, J.; Zeng, M.; Xu, J.; Wang, X.; Hu, W. Nanoscale 2014, 6, 4157.
doi: 10.1039/c3nr06744k |
| [15] | Shi, B.; Su, Y.; Zhang, L.; Huang, M.; Liu, R; Zhao, S. . ACS Appl. Mater. Interfaces 2016, 8, 10717. |
| [16] | Han, Y.; Wu, X.; Zhang, X.; Zhou, Z.; Lu, C. ACS Sustainable Chem. Eng. 2016, 4, 5667. |
| [17] |
Khadgi, N.; Upretia, A. R.; Li, Y. RSC Adv. 2017, 7, 27007.
doi: 10.1039/C7RA01782K |
| [18] | Zhang, Y.-Y.; Chen, X.-Z.; Liu, X.-Y.; Wang, M.; Liu, J.-J.; Gao, G.; Zhang, X.-Y.; Sun, R.-Z.; Hou, S.-C.; Wang, H.-M . Sens. Actuators, B. 2018, 273, 1077. |
| [19] |
Sakunkaewkasem, S.; Petdum, A.; Panchan, W.; Sirirak, J.; Charoenpanich, A.; Sooksimuang, T.; Wanichacheva, N. ACS Sens. 2018, 3, 1016.
doi: 10.1021/acssensors.8b00158 |
| [20] | Xia, Q.; Chen, Z.; Zhang, Z.; Liu, R. Chin. J. Org. Chem. 2018, 38, 2700 (in Chinese). |
| ( 夏琦, 陈子康, 张志德, 刘瑞源, 有机化学, 2018, 38, 2700.) | |
| [21] | Wu, Y.-C.; Jiang, K.; Luo, S.-H.; Cao, L.; Wu, H.-Q.; Wang, Z.-Y. Spectrochim. Acta, Part A 2019, 206, 632. |
| [22] | Xu, Z.-H.; Wang, Y.; Wang, Y.; Li, J.-Y.; Luo, W.-F.; Wu, W.-N.; Fan, Y.-C. Spectrochim. Acta, Part A 2019, 212, 146. |
| [23] | Wang, Z.; Yang, J.; Yang, Y.; Xu, X.; Li, M.; Zhang, Y.; Fang, H.; Xu, H.; Wang, S. Chin. J. Org. Chem. 2018, 38, 1401 (in Chinese). |
| ( 王忠龙, 杨金来, 杨益琴, 徐徐, 李明新, 张燕, 方华, 徐海军, 王石发, 有机化学 , 2018, 38, 1401.) | |
| [24] |
Shekari, Z.; Younesi, H.; Heydari, A.; Tajbakhsh, M.; Chaichi, M.; Shahbazi, J. A.; Saberi, D. Chemosensors 2017, 5, 26.
doi: 10.3390/chemosensors5030026 |
| [25] | Shi, B. B.; Zhang, P.; Wei, T.-B.; Yao, H.; Qi, L.; Liu, J.; Zhang, Y.-M. Tetrahedron 2013, 16. 7981. |
| [26] | Wang, L.-Y.; Fang, G.-P.; Cao, D.-R. Sens. Actuators, B 2015, 207, 849. |
| [27] |
Abrego, Z.; Unceta, N.; Sanchez, A.; Gomez-Caballero, A.; Berrio-Ochoa, L. M.; Goicolea, M. A.; Barrio, J. Analyst 2017, 142, 1157.
doi: 10.1039/C7AN00049A |
| [28] | Asadpour-Zeynali, K.; Amini, R. Sens. Actuators, B 2017, 246, 961. |
| [29] | Kallithrakas-Kontos, N.; Foteinis, S.C. Anal. Chem. 2016, 12, 22. |
| [30] | Song, C. Y.; Yang, B. Y.; Yang, Y. J.; Wang, L.H. Sci. China: Chem. 2016, 59, 16. |
| [31] | Sun, Z. L.; Du, J. J; Jing, C.Y. . J. Environ. Sci. 2016, 39, 134. |
| [32] | West, M.; Ellis, A. T.; Potts, P. J.; Streli, C.; Vanhoof, C.; Wobrauschek, P. J. Anal. At. Spectrom. 2016, 31, 1706. |
| [33] | van den, Berg. C. M. G. , Anal. Chem. 2006, 78, 156. |
| [34] |
Lunvongsa, S.; Oshima, M.; Motomizu, S. Talanta 2006, 68, 969.
doi: 10.1016/j.talanta.2005.06.067 |
| [35] | Graser, C. H.; Banik, N. I.; Bender, K. A.; Lagos, M.; Marquardt, C. M.; Marsac, R.; Montoya, V.; Geckeis, H. Anal. Chem. 2015, 87, 9786. |
| [36] | Liu, R.; Zeng, J. Chin. J. Org. Chem. 2017, 37, 3274 (in Chinese). |
| ( 刘瑞姣, 曾竟, 有机化学 , 2017, 37, 3274.) | |
| [37] | Zhang, M.; Xiao, H.; Han, Z.; Yang, L.; Wu, X. Chin. J. Org. Chem. 2018, 38, 926 (in Chinese). |
| ( 张敏, 肖慧丰, 韩志湘, 仰榴青, 吴向阳, 有机化学, 2018, 38, 926.) | |
| [38] |
Zhang, H.; Nie, C.; Wang, J.; Guan, R.; Cao, D. Talanta 2019, 195, 713.
doi: 10.1016/j.talanta.2018.11.082 |
| [39] |
Behera, K. C.; Bag, B. Dyes Pigm. 2016, 135, 143.
doi: 10.1016/j.dyepig.2016.04.034 |
| [40] | Zhang, X.; Man Lai, E. S.; Martin-Aranda, R.; Yeung, K.L. Appl. Catal. A 2004, 261, 109. |
| [41] | Chen, Z.; Cai, D.; Mou, D.; Yan, Q.; Sun, Y.; Pan, W.; Wan, Y.; Song, H.; Yi, W. Bioorg. Med. Chem. 2014, 22, 3279. |
| [42] | Wang, J.; Zhang, L.; Qi, Q.; Li, S.; Jiang, Y. Anal. Methods 2013, 5, 608. |
| [43] | Zhang, Y.-M.; Zhu, W.; Qu, W.-J.; Zhong, K.-P.; Chen, X.-P.; Yao, H.; Wei, T.-B.; Lin, Q. Chem. Commun. 2018, 54, 4549. |
| [44] | Qi, L.; Fan, Y.-Q.; Gong, G.-F.; Mao, P.-P.; Wang, J.; Guan, X.-W.; Liu, J.; Zhang, Y.-M.; Yao, H.; Wei, T.-B. ACS Sustainable Chem. Eng. 2018, 6, 8775. |
| [45] | Li, X.; Lin, Q.; Qu, W-J.; Li, Q.; Chen, X.-B.; Li, W.-T.; Zhang, Y.-M.; Yao, H.; Wei, T.-B. Chin. J. Org. Chem. 2017, 37, 889 (in Chinese). |
| ( 李翔, 林奇, 曲文娟, 李乔, 程晓斌, 李文婷, 张有明, 姚虹, 魏太保, 有机化学, 2017, 37, 889.) | |
| [46] | Li, W.-T.; Qu, W-J.; Zhang, H.-L.; Li, X.; Lin, Q.; Yao, H.; Zhang, Y.-M.; Wei, T.-B. Chin. J. Org. Chem. 2017, 37, 2619 (in Chinese). |
| ( 李文婷, 曲文娟, 张海丽, 李翔, 林奇, 姚虹, 张有明, 魏太保, 有机化学, 2017, 37, 2619.) | |
| [47] |
Ma, X.-Q.; Wang, Y.; Wei, T.-B.; Qi, L.-H.; Jiang, X.-M.; Ding, J.-D.; Zhu, W.-B.; Yao, H.; Zhang, Y.-M.; Lin, Q. Dyes Pigm. 2019, 164, 279.
doi: 10.1016/j.dyepig.2019.01.049 |
| [48] | Kim, H. N.; Ren, W. X.; Kim, J. S.; Yoon, J. Chem. Soc. Rev. 2012, 41, 3210. |
| [49] | Shi, W.; Zhao, S.; Su, Y.; Hui, Y.; Xie, Z.F. New J. Chem. 2016, 40, 7814. |
| [50] | Lin, L.; Lv, J.; Ji, Y.; Feng, J.; Liu, Y.; Wang, Z.; Zhang, W. Anal. Lett. 2013, 46, 2890. |
| [51] | Wang, L.; Zeng, R.; Li, C.; Qiao, R. Colloids Surf., B 2009, 74, 284. |
| [1] | Huanqing Li, Zhaohua Chen, Zujia Chen, Qiwen Qiu, Youcai Zhang, Sihong Chen, Zhaoyang Wang. Research Progress in Mercury Ion Fluorescence Probes Based on Organic Small Molecules [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3067-3077. |
| [2] | Yangyang Li, Xiaofei Sun, Xiaoling Hu, Yuanyuan Ren, Keli Zhong, Xiaomei Yan, Lijun Tang. Synthesis of Triphenylamine Derivative and Its Recognition for Hg2+ with “OFF-ON” Fluorescence Response Based on Aggregation-Induced Emission (AIE) Mechanism [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 320-325. |
| [3] | Ma Xuelin, Han Limin, Zhang Xiaoyong, Hao Zhanzhong, Yang Wei, Zhang Yuheng, Wang Li. A Highly Stable Multi-response Zirconium(IV) Metal-Organic Frameworks for Fluorescence Sensing of Fe3+, Cr2O72- and Organic Small Molecules [J]. Chinese Journal of Organic Chemistry, 2020, 40(9): 2938-2948. |
| [4] | Cao Xiying, Luo Shihe, Yang Chongling, Xiao Ying, Li Xiaoyan, Zhang Junru, Wang Zhaoyang. Research Progress of Organic Polymer Fluorescence Sensor [J]. Chinese Journal of Organic Chemistry, 2020, 40(12): 4046-4059. |
| [5] | Liu, Yu, Tang, Yongxing, Luo, Yueyang, Zhu, Yeting, Yang, Jin, Wei, Xinyu, Liu, Xiong, Zhao, Yunhui. Synthesis and Application of Fluorescent Probe Containing Barbitone Unit [J]. Chinese Journal of Organic Chemistry, 2019, 39(10): 2980-2984. |
| [6] | Fu Yi, Tang Hui, Liu Ze, Zhang Wanxuan, Ren Jun. Synthesis and Properties of a Fluoride Ion Fluorescence Chemosensor [J]. Chin. J. Org. Chem., 2018, 38(7): 1806-1810. |
| [7] | Li Xiang, Lin Qi, Qu Wenjuan, Li Qiao, Chen Xiaobin, Li Wenting, Zhang Youming, Yao Hong, Wei Taibao. A Novel Imidazophenazine Lactam Reaction Type Recognition Cyanide Ion Fluorescence Probe [J]. Chin. J. Org. Chem., 2017, 37(4): 889-895. |
| [8] | Xiao Liwei, Ren Ping, Jing Xuemin, Ren Lilei, Li Zheng, Dai Fucai. Application in Molecular Recognition of 1,2,3-Trizole Derivatives [J]. Chin. J. Org. Chem., 2017, 37(12): 3085-3095. |
| [9] | Zhu Jianghua, Fan Xiangtian, Jiang Ping, Cao Qianyong. A Tripodal Anthracene-Appended Triazole for Fluorescence Sensing of Hg2+ [J]. Chin. J. Org. Chem., 2017, 37(1): 185-189. |
| [10] | Xiao Huifeng, Zhang Min, Liu Jie, Han Zhixiang, Yang Liuqing, Wu Xiangyang. A Novel Rhodamine B Fluorescent Probe for Hg2+: Synthesis and Evaluation [J]. Chin. J. Org. Chem., 2016, 36(10): 2413-2418. |
| [11] | Sun Jingfu, Qian Ying. A Novel Naphthalimide-Rhodamine Fluorescence Sensor: Synthesis, Aggregation-Induced Emission Enhancement and Its Dual-Channel Detection Property [J]. Chin. J. Org. Chem., 2016, 36(1): 151-157. |
| [12] | Su Na, Yang Meipan, Meng Wenfei, Yang Bingqin. Synthesis and Properties Characterization of Novel Fe3+ Fluorescent Probe Based on Rhodamine B [J]. Chin. J. Org. Chem., 2015, 35(1): 175-180. |
| [13] | Zhou Jia, Yang Meipan, Meng Wenfei, Cheng Zhao, Yang Bingqin. Synthesis of a Reversible Fluorescence Probe for Hg2+ and Its Application in Living Cells [J]. Chin. J. Org. Chem., 2014, 34(8): 1646-1651. |
| [14] | Meng Wenfei, Yang Meipan, Cheng Zhao, Li Shaoni, Yang Bingqin. Synthesis of Novel Rhodamine B Fluorescent Probe and Recognition Study to Fe3+ [J]. Chin. J. Org. Chem., 2014, 34(2): 398-402. |
| [15] | WU Zhong-Yu,FANG Hao*,XU Wen-Fang. Progress in Boronic Acid-Based Fluorescent Glucose Sensors [J]. Chin. J. Org. Chem., 2007, 27(07): 830-836. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||