Chinese Journal of Organic Chemistry ›› 2019, Vol. 39 ›› Issue (11): 3294-3298.DOI: 10.6023/cjoc201903007 Previous Articles Next Articles
Special Issue: 碳氢活化合辑2018-2019
谢庭辉ab, 蒋筱莹a, 米治胜a, 李雪a, 徐小河a, 白仁仁a, 帅棋a, 谢媛媛a*( )
)
收稿日期:2019-03-04
发布日期:2019-07-09
通讯作者:
谢媛媛
E-mail:xyycz@zjut.edu.cn
基金资助:
Xie Tinghuiab, Jiang Xiaoyinga, Mi Zhishenga, Li Xuea, Xu Xiaohea, Bai Renrena, Shuai Qia, Xie Yuanyuana*( )
)
Received:2019-03-04
Published:2019-07-09
Contact:
Xie Yuanyuan
E-mail:xyycz@zjut.edu.cn
Supported by:Share
Xie Tinghui, Jiang Xiaoying, Mi Zhisheng, Li Xue, Xu Xiaohe, Bai Renren, Shuai Qi, Xie Yuanyuan. Iron/O2-Promoted C-H Bond Functionalization for the Exclusive Synthesis of 2-Quinoline Carboxaldehydes under Microwave Irradiation[J]. Chinese Journal of Organic Chemistry, 2019, 39(11): 3294-3298.
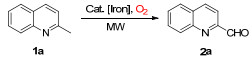 | ||||||
| Entry | Promoter | Fe3+/1a (molar ratio) | Solvent | Temp./℃ | Timeb/min | Yieldc/% |
| 1 | FeCl2 | 1/1 | DMSO | 130 | 30 | Trace |
| 2 | FeCl3 | 1/1 | DMSO | 130 | 10 | 60 |
| 3 | FeCl3·6H2O | 1/1 | DMSO | 130 | 10 | 62 |
| 4 | Fe(NO3)3·9H2O | 1/1 | DMSO | 130 | 10 | 65 |
| 5 | Fe(NO3)3·9H2O | 0.5/1 | DMSO | 130 | 10 | 46 |
| 6 | Fe(NO3)3·9H2O | 1.5/1 | DMSO | 130 | 10 | 58 |
| 7 | Fe(NO3)3·9H2O | 1/1 | DMF | 130 | 15 | 61 |
| 8 | Fe(NO3)3·9H2O | 1/1 | Toluene | 130 | 30 | 53 |
| 9 | Fe(NO3)3·9H2O | 1/1 | Dioxane | 130 | 30 | 48 |
| 10 | Fe(NO3)3·9H2O | 1/1 | DMAC | 130 | 15 | 60 |
| 11 | Fe(NO3)3·9H2O | 1/1 | DMSO | 140 | 8 | 70 |
| 12 | Fe(NO3)3·9H2O | 1/1 | DMSO | 150 | 5 | 78 |
| 13 | Fe(NO3)3·9H2O | 1/1 | DMSO | 160 | 5 | 71 |
| 14 | Fe(NO3)3·9H2O | 1/1 | DMSO | 150 | 1 | 37 |
| 15 | Fe(NO3)3·9H2O | 1/1 | DMSO | 150 | 10 | 76 |
| 16d | Fe(NO3)3·9H2O | 1/1 | DMSO | 150 | 30 | 45 |
| 17e | Fe(NO3)3·9H2O | 1/1 | DMSO | 150 | 30 | 18 |
 | ||||||
| Entry | Promoter | Fe3+/1a (molar ratio) | Solvent | Temp./℃ | Timeb/min | Yieldc/% |
| 1 | FeCl2 | 1/1 | DMSO | 130 | 30 | Trace |
| 2 | FeCl3 | 1/1 | DMSO | 130 | 10 | 60 |
| 3 | FeCl3·6H2O | 1/1 | DMSO | 130 | 10 | 62 |
| 4 | Fe(NO3)3·9H2O | 1/1 | DMSO | 130 | 10 | 65 |
| 5 | Fe(NO3)3·9H2O | 0.5/1 | DMSO | 130 | 10 | 46 |
| 6 | Fe(NO3)3·9H2O | 1.5/1 | DMSO | 130 | 10 | 58 |
| 7 | Fe(NO3)3·9H2O | 1/1 | DMF | 130 | 15 | 61 |
| 8 | Fe(NO3)3·9H2O | 1/1 | Toluene | 130 | 30 | 53 |
| 9 | Fe(NO3)3·9H2O | 1/1 | Dioxane | 130 | 30 | 48 |
| 10 | Fe(NO3)3·9H2O | 1/1 | DMAC | 130 | 15 | 60 |
| 11 | Fe(NO3)3·9H2O | 1/1 | DMSO | 140 | 8 | 70 |
| 12 | Fe(NO3)3·9H2O | 1/1 | DMSO | 150 | 5 | 78 |
| 13 | Fe(NO3)3·9H2O | 1/1 | DMSO | 160 | 5 | 71 |
| 14 | Fe(NO3)3·9H2O | 1/1 | DMSO | 150 | 1 | 37 |
| 15 | Fe(NO3)3·9H2O | 1/1 | DMSO | 150 | 10 | 76 |
| 16d | Fe(NO3)3·9H2O | 1/1 | DMSO | 150 | 30 | 45 |
| 17e | Fe(NO3)3·9H2O | 1/1 | DMSO | 150 | 30 | 18 |
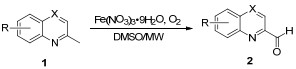 | ||||||
| Entry | X | R | Timeb/min | Fe3+/1 (molar ratio) | Product | Yieldc/% |
| 1 | CH | H | 5 | 1.0 | 2a | 78 |
| 2 | CH | 6-OMe | 10 | 1.5 | 2b | 60 |
| 3 | CH | 7-OMe | 10 | 1.5 | 2c | 64 |
| 4 | CH | 3-Me | 10 | 1.5 | 2d | 54 |
| 5 | CH | 6-Me | 10 | 1.5 | 2e | 63 |
| 6 | CH | 8-Me | 10 | 1.5 | 2f | 48 |
| 7 | CH | 8-Cl | 5 | 1.0 | 2g | 52 |
| 8 | CH | 6-NO2 | 5 | 1.0 | 2h | 81 |
| 9 | CH | 6-F | 5 | 1.0 | 2i | 73 |
| 10 | CH | 6-Cl | 5 | 1.0 | 2j | 67 |
| 11 | N | H | 5 | 1.0 | 2k | 80 |
 | ||||||
| Entry | X | R | Timeb/min | Fe3+/1 (molar ratio) | Product | Yieldc/% |
| 1 | CH | H | 5 | 1.0 | 2a | 78 |
| 2 | CH | 6-OMe | 10 | 1.5 | 2b | 60 |
| 3 | CH | 7-OMe | 10 | 1.5 | 2c | 64 |
| 4 | CH | 3-Me | 10 | 1.5 | 2d | 54 |
| 5 | CH | 6-Me | 10 | 1.5 | 2e | 63 |
| 6 | CH | 8-Me | 10 | 1.5 | 2f | 48 |
| 7 | CH | 8-Cl | 5 | 1.0 | 2g | 52 |
| 8 | CH | 6-NO2 | 5 | 1.0 | 2h | 81 |
| 9 | CH | 6-F | 5 | 1.0 | 2i | 73 |
| 10 | CH | 6-Cl | 5 | 1.0 | 2j | 67 |
| 11 | N | H | 5 | 1.0 | 2k | 80 |
| [1] |
(a) Li, Z.; Wu, S.-S.; Luo, Z.-G.; Liu, W.-K.; Feng, C.-T.; Ma, S.-T. J. Org. Chem. 2016, 81, 4386.
doi: 10.1021/acs.joc.6b00569 |
|
(b) Xu, L.-B.; Shao, Z.-Z.; Wang, L.; Zhao, H.-L.; Xiao, J. Tetrahedron Lett. 2014, 55, 6856.
doi: 10.1021/acs.joc.6b00569 |
|
|
(c) Thirunavukkarasu, V. S.; Kozhushkov S. I.; Ackermann, L. Chem. Commun. 2014, 50, 29.
doi: 10.1021/acs.joc.6b00569 |
|
| [2] |
(a) Derong, D.; Linda, P. D.; Peter, A. C. Tetrahedron Lett. 2013. 54, 5211.
doi: 10.1016/j.tetlet.2013.07.067 |
|
(b) Jiang, L.; Huang, Y.-Y.; Yan, Y.-Y.; Xie, Y.-Y. Tetrahedron Lett. 2016, 57, 4149.
doi: 10.1016/j.tetlet.2013.07.067 |
|
| [3] |
Guo S.-J. Wan G. Sun S. Jiang Y. Yu J.-T. Cheng J. Chem. Commun. 2015 51 5085.
doi: 10.1039/C5CC01024A |
| [4] |
Xie Y.-Y. Li L.-H. Tetrahedron Lett. 2014 55 3892.
doi: 10.1016/j.tetlet.2014.04.023 |
| [5] |
(a) Adsule, S.; Barve, V.; Chen, D.; Ahmed, F.; Dou, Q. P.; Padhye, S.; Sarkar, F. H. J. Med. Chem. 2006, 49, 7242.
doi: 10.1021/jm060712l |
|
(b) Teguh, S. C.; Klonis, N.; Duffy, S.; Lucantoni, L.; Avery, V. M.; Hutton, C. A.; Baell, J. B.; Tilley, L. J. Med. Chem. 2013, 56, 6200.
doi: 10.1021/jm060712l |
|
| [6] |
(a) Pokhrel, L.; Kim, Y.; Nguyen, T. D. T.; Prior, A. M.; Lu, J. Y.; Chang, K. O.; Hua, D. H. Bioorg. Med. Chem. Lett. 2012, 22, 3480.
doi: 10.1016/j.bmcl.2012.03.084 |
|
(b) Dai, Q.; Yu, J.-T.; Feng, X.-M.; Jiang, Y.; Yang, H.-T.; Cheng, J. Adv. Synth. Catal. 2014, 356, 3341.
doi: 10.1016/j.bmcl.2012.03.084 |
|
|
(c) Gopinath, V. S.; Pinjari, J.; Dere, R. T.; Verma, A.; Vishwakarma, P.; Shivahare, R.; Moger, M.; Goud, P. S. K.; Ramanathan, V.; Bose, P.; Rao, M. V. S.; Gupta, S.; Puri, S. K.; Launay, D.; Martin, D. Eur. J. Med. Chem. 2013, 69, 527.
doi: 10.1016/j.bmcl.2012.03.084 |
|
| [7] | Holzapfel C. W. Ferreira A. C. Marais W. J. Chem. Res., Synop. 2002 5 218. |
| [8] |
Ding D. Dwoskin L. P. Crooks P. A. Tetrahedron Lett. 2013 54 5211.
doi: 10.1016/j.tetlet.2013.07.067 |
| [9] |
Minisci F. Vismara E. Levi S. J. Org. Chem. 1986 51 536.
doi: 10.1021/jo00354a026 |
| [10] |
Zheng G. Liu H. Wang M. Chin. J. Chem. 2016 34 519.
doi: 10.1002/cjoc.201500918 |
| [11] |
(a) Sindhu, K. S.; Abi, T. G.; Mathai, G.; Anilkumar, G. Polyhedron 2019, 158, 270.
doi: 10.6023/cjoc201806007 |
|
(b) Wusiman, A.; Hudabaierdi, R. Tetrahedron Lett. 2019, 60, 681.
doi: 10.6023/cjoc201806007 |
|
|
(c) Wang, X.-Z.; Zeng, C.-C. Tetrahedron 2019, 75, 1425.
doi: 10.6023/cjoc201806007 |
|
|
(d) Ding, X.-Y.; Xu, F. Chin. J. Org. Chem. 2018, 38, 3345 (in Chinese).
doi: 10.6023/cjoc201806007 |
|
|
(丁晓友, 徐凡, 有机化学, 2018, 38, 3345.)
doi: 10.6023/cjoc201806007 |
|
| [12] |
(a) Ma, S.-M.; Liu, J.-X.; Li, S.-H.; Chen, B.; Cheng, J.-J.; Kuang, J.-Q.; Liu, Y.; Wan, B.-Q.; Wang, Y.-L.; Ye, J.-T.; Yu, Q.; Yuan, W. M.; Yu, S.-C. Adv. Synth. Catal. 2011, 353, 1005.
doi: 10.1002/adsc.201100033 |
|
(b) Silva, M. J.; Carari, D. M. Catal. Lett. 2014, 144, 615.
doi: 10.1002/adsc.201100033 |
|
|
(c) Tanaka, S.; Kon, Y.; Uesaka, Y.; Morioka, R.; Tamura, M.; Sato, K. Chem. Lett. 2016, 45, 188.
doi: 10.1002/adsc.201100033 |
|
|
(d) Xu, X.-H.; Sun, J.; Cheng, J.-Y.; Li, P.-P.; Jiang, X.-Y.; Bai, R.-R.; Xie, Y.-Y. Eur. J. Org. Chem. 2017, 47, 7160.
doi: 10.1002/adsc.201100033 |
|
|
(e) Amaya, T.; Fujimoto, H. Tetrahedron Lett. 2018, 59, 2657.
doi: 10.1002/adsc.201100033 |
|
|
(f) Wen, J.; Zhang, J.; Chen, S.-Y.; Li, J.; Yu, X.-Q. Angew. Chem. Int. Ed. 2008, 47, 8897.
doi: 10.1002/adsc.201100033 |
|
|
(g) Namboodiri, V. V.; Polshettiwar, V.; Varma, R. S. Tetrahedron Lett. 2007, 48, 8839.
doi: 10.1002/adsc.201100033 |
|
| [13] |
(a) Jiang, K.; Pi, D.-W.; Zhou, H.-F.; Liu, S.-S.; Zou, K. Tetrahedron. 2014, 70, 3056.
doi: 10.1016/j.tet.2014.02.069 |
|
(b) Rao, N. N.; Meshram, H. M.; Tetrahedron Lett. 2013, 54, 1315.
doi: 10.1016/j.tet.2014.02.069 |
|
|
(c) Li, Q.; Huang, Y.; Chen, T.-Q.; Zhou, Y.-B.; Xu, Q.; Yin, S.-F.; Han, L.-B. Org. Lett. 2014, 16, 3672.
doi: 10.1016/j.tet.2014.02.069 |
|
| [14] | Yasunari M. Kanoko Y. Takashi T. Takayuki A. Tomohiro M. Kosaku H. Hironao S. Heterocycles 2010 80 737. |
| [15] |
Mathes W. Sauermilch W. Chem. Ber. 1957 90 758.
doi: 10.1002/cber.19570900519 |
| [16] | Chen X.-Y. Shi J. Li Y.-M. Wang F.-L. Wu X. Guo Q.-X. Liu L. Org. Lett. 2009 11 4421. |
| [17] |
Brown B. R. Hammick D. L. J. Chem. Soc. 1950 628.
doi: 10.1039/jr9500000628 |
| [18] |
Buehler C. A. Edwards S. P. J. Am. Chem. Soc. 1952 74 4 977.
doi: 10.1021/ja01124a032 |
| [19] |
Ballesteros G. R. Leroux F. R. Ballesteros R. Tetrahedron 2009 65 22 4410.
doi: 10.1016/j.tet.2009.03.058 |
| [20] | Janina, B. PL 56421, 1968[Chem. Abstr. 1968, 70, 115023]. |
| [21] | Wang L. Hou X.-B. Fu H.-S. Pan X.-L. Xu W.-F. Tang W.-P Fang H. Bioorg. Med. Chem. 2015 15 23 4364. |
| [22] |
Leese C. L. Rydon H. N. J. Chem. Soc. 1956 303.
doi: 10.1039/jr9560000303 |
| [1] | Kaikai Wang, Shaowei Chen, Yajun Li, Daliang Li, Hongli Bao. Iron-Catalyzed Decarboxylative Heck-Type Alkylation of Conjugate 1,3-Dienes [J]. Chinese Journal of Organic Chemistry, 2021, 41(7): 2707-2714. |
| [2] | Linyang Wu, Dayou Zhong, Wenbo Liu. Ligand-Free Iron-Catalyzed Intramolecular Amination of C(sp3)—H Bond for the Synthesis of Imidazolinones [J]. Chinese Journal of Organic Chemistry, 2021, 41(10): 4083-4087. |
| [3] | Abdukader Ablimit, Wang Rong, Mamat Marhaba, Liu Chenjiang. FeCl2-Catalyzed Intramolecular Aerobic Reaction for Construction of Isoxazoles Heterocycle [J]. Chinese Journal of Organic Chemistry, 2020, 40(6): 1697-1703. |
| [4] | Sun Qiangsheng, Sun Wei. Recent Progress in C(sp3)-H Asymmetric Oxidation Catalyzed by Bioinspired Metal Complexes [J]. Chinese Journal of Organic Chemistry, 2020, 40(11): 3686-3696. |
| [5] | He Zeyu, Fan Min, Xu Jia'neng, Hu Yue, Wang Lu, Wu Xudong, Xia Chungu, Liu Chao. Iron-Catalyzed Deoxygenative Diborylation of Ketones to Internal gem-Diboronates [J]. Chinese Journal of Organic Chemistry, 2019, 39(12): 3438-3445. |
| [6] | Zong Chaoyang, Gu Huiwen, Zhang Lijie, Jin Yudong, Sun Yaquan. Microwave-Accelerated Dimroth Rearrangement for the Synthesis of Pyrido [2, 3-d]pyrimidin-4-amine Derivatives [J]. Chin. J. Org. Chem., 2018, 38(5): 1165-1171. |
| [7] | Qu Renyu, Chen Nian, Liu Yuchao, Chen Qiong, Yang Guangfu. An Efficient Synthesis of Functionalized 6-Arylsubstituted Salicylates via Microwave Irradiation [J]. Chin. J. Org. Chem., 2017, 37(5): 1266-1272. |
| [8] | Shi Dongdong, Bao Hanyang, Xu Zheng, Liu Yunkui. Synthesis of 6-Aryl Phenanthridines via Iron-Catalyzed sp2-C-H Bond Amination/Aromatization Reaction [J]. Chin. J. Org. Chem., 2017, 37(5): 1290-1294. |
| [9] | Huang Yaobing, Yang Tao, Liu Anfeng, Zhou Xincheng, Pan Hui. Microwave-Assisted Alcoholysis of Cellulose to Methyl Levulinate Catalyzed by SnCl4/H2SO4 [J]. Chin. J. Org. Chem., 2016, 36(6): 1438-1443. |
| [10] | Zhang Bianxiang, Yang Lihua, Shi Ruixue, Kang Yongqiang. Synthesis of Heterocyclic Aromatic Sulfides under Microwave Irradiation [J]. Chin. J. Org. Chem., 2016, 36(2): 352-357. |
| [11] | Zhang Rui, Pan Jinhuan, Wu Qiongyou. Copper Mediated One-Pot Multi-Component Synthesis of 3-Substitued Isoquinolines [J]. Chin. J. Org. Chem., 2016, 36(12): 2906-2911. |
| [12] | Zhu Jihua, Zheng Xudong, Guo Guozhe, Zhang Yuquan, Wu Bowan. Microwave-Assisted Synthesis of Asymmetrical 1,5-Disubstituted Carbonohydrazide and Crystal Structure [J]. Chin. J. Org. Chem., 2015, 35(9): 1975-1980. |
| [13] | Feng Junna, Li Xiaohui, Shao Jie, Zhu Mo, Li Yan, Chen Hua, Li Xiaoliu. Microwave Assisted Synthesis and Anti-HIV-RT Activity of Diaryl Benzo[1,3]thiazin-4-ones [J]. Chin. J. Org. Chem., 2015, 35(6): 1370-1374. |
| [14] | Wang Juxian, Lin Wei, Liu Hongtao, Hu Minghua, Feng Xian, Ren Jinfeng, Huang Zhibin, Shi Daqing. An Efficient Synthesis of Coumarino[4,3-d]pyrazolo[3,4-b]-pyridine Derivatives Catalyzed by Silica Sulfuric Acid under Microwave Irradiation [J]. Chin. J. Org. Chem., 2015, 35(4): 927-933. |
| [15] | Li Yingjun, Shi Xiangling, Gao Lixin, Jin Kun, Sheng Li, Wu Jianghong, Peng Lina, Li Jia. Synthesis, Characterization and Biological Activities of 3,5,6-Trisubstituted-1,2,4-triazine Derivatives [J]. Chin. J. Org. Chem., 2015, 35(1): 191-199. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||