Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (5): 1897-1924.DOI: 10.6023/cjoc202009053 Previous Articles Next Articles
REVIEWS
收稿日期:2020-09-27
修回日期:2020-11-14
发布日期:2020-12-01
通讯作者:
俞静波
基金资助:
Hao Wanga, Ping Yingb, Jingbo Yua,*( ), Weike Sua,b
), Weike Sua,b
Received:2020-09-27
Revised:2020-11-14
Published:2020-12-01
Contact:
Jingbo Yu
About author:Supported by:Share
Hao Wang, Ping Ying, Jingbo Yu, Weike Su. Alternative Strategies Enabling Cross-Dehydrogenative Coupling: Access to C—C Bonds[J]. Chinese Journal of Organic Chemistry, 2021, 41(5): 1897-1924.

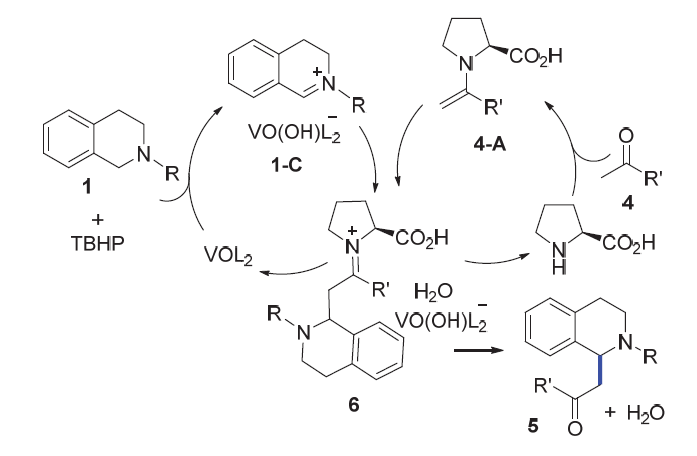
| [1] |
Li, C.-J.; Li, Z. P. Pure Appl. Chem. 2006, 78, 935.
doi: 10.1351/pac200678050935 |
| [2] |
Li, C.-J. Acc. Chem. Res. 2009, 42, 335.
doi: 10.1021/ar800164n |
| [3] |
Guo, X. W.; Li, Z. P.; Li, C. J. Prog. Chem. 2010, 22, 1434. (in Chinese).
|
|
(郭兴伟, 李志平, 李朝军, 化学进展, 2010, 22, 1434.)
|
|
| [4] |
Girard, S. A.; Knauber, T.; Li, C.-J. Angew. Chem., Int. Ed. 2014, 53, 74.
|
| [5] |
Huang, C.-Y.; Kang, H.; Li, J.; Li, C.-J. J. Org. Chem. 2019, 84, 12705.
doi: 10.1021/acs.joc.9b01704 |
| [6] |
Zhang, Y.; Feng, B. N. Chin. J. Org. Chem. 2014, 34, 2406. (in Chinese).
doi: 10.6023/cjoc201408030 |
|
(张艳, 冯柏年, 有机化学, 2014, 34, 2406.)
doi: 10.6023/cjoc201408030 |
|
| [7] |
Phillips, A. M. F.; Pombeiro, A. J. L. ChemCatChem 2018, 10, 3354.
doi: 10.1002/cctc.201800582 |
| [8] |
Parvatkar, P. T.; Manetsch, R.; Banik, B. K. Chem.-Asian J. 2019, 14, 6.
doi: 10.1002/asia.201801237 |
| [9] |
Lei, A. Transition Metal Catalyzed Oxidative Cross-Coupling Reactions, Springer, Berlin,2019.
|
| [10] |
Scheuermann, C. J. Chem.-Asian J. 2010, 5, 436.
doi: 10.1002/asia.v5:3 |
| [11] |
Varun, B. V.; Dhineshkumar, J.; Bettadapur, K. R.; Siddaraju, Y.; Alagiri, K.; Prabhu, K. R. Tetrahedron Lett. 2017, 58, 803.
doi: 10.1016/j.tetlet.2017.01.035 |
| [12] |
Revathi, L.; Ravindar, L.; Fang, W.-Y.; Rakesh, K. P.; Qin, H.-L. Adv. Synth. Catal. 2018, 360, 4652.
doi: 10.1002/adsc.201800736 |
| [13] |
Chen, B.; Wu, L.-Z.; Tung, C.-H. Acc. Chem. Res. 2018, 51, 2512.
doi: 10.1021/acs.accounts.8b00267 |
| [14] |
Jiang, C.; Chen, W.; Zheng, W.-H.; Lu, H. Org. Biomol. Chem. 2019, 17, 8673.
doi: 10.1039/C9OB01609K |
| [15] |
Wang, H.; Gao, X.; Lv, Z.; Abdelilah, T.; Lei, A. Chem. Rev. 2019, 119, 6769.
doi: 10.1021/acs.chemrev.9b00045 |
| [16] |
Tang, S.; Liu, Y.; Lei, A. Chem 2018, 4, 27.
|
| [17] |
(a) Dong, K.; Liu, Q.; Wu, L.-Z. Acta Chim. Sinica 2020, 78, 299. (in Chinese).
doi: 10.6023/A19110412 |
|
(董奎, 刘强, 吴骊珠, 化学学报, 2020, 78, 299.)
doi: 10.6023/A19110412 |
|
|
(b) Kong, Y. L.; Xu, W. X.; Ye, F. X.; Weng, J. Q. Chin. J. Org. Chem. 2019, 39, 3065. (in Chinese).
doi: 10.6023/cjoc201905016 |
|
|
(孔瑶蕾, 徐雯秀, 叶飞霞, 翁建全, 有机化学, 2019, 39, 3065.)
doi: 10.6023/cjoc201905016 |
|
|
(c) Wu, Y.; Xi, Y.; Zhao, M.; Wang, S. Chin. J. Org. Chem. 2018, 38, 2590. (in Chinese).
doi: 10.6023/cjoc201804001 |
|
|
(吴亚星, 席亚超, 赵明, 王思懿, 有机化学, 2018, 38, 2590.)
doi: 10.6023/cjoc201804001 |
|
|
(d) Zhong, J.-J.; Meng, Q.-Y.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Acta Chim. Sinica 2017, 75, 34. (in Chinese).
doi: 10.6023/A16090491 |
|
|
(钟建基, 孟庆元, 陈斌, 佟振合, 吴骊珠, 化学学报, 2017, 75, 34.)
doi: 10.6023/A16090491 |
|
| [18] |
Yuan, Y.; Lei, A. Acc. Chem. Res. 2019, 52, 3309.
doi: 10.1021/acs.accounts.9b00512 |
| [19] |
Rodriguez, B.; Bruckmann, A.; Rantanen, T.; Bolm, C. Adv. Synth. Catal. 2007, 349, 2213.
doi: 10.1002/(ISSN)1615-4169 |
| [20] |
Lou, S.-J.; Mao, Y.-J.; Xu, D.-Q.; He, J.-Q.; Chen, Q.; Xu, Z.-Y. ACS Catal. 2016, 6, 3890.
doi: 10.1021/acscatal.6b00861 |
| [21] |
Hernandez, J. G.; Bolm, C. J. Org. Chem. 2017, 82, 4007.
doi: 10.1021/acs.joc.6b02887 |
| [22] |
Egorov, I. N.; Santra, S.; Kopchuk, D. S.; Kovalev, I. S.; Zyryanov, G. V.; Majee, A.; Ranu, B. C.; Rusinov, V. L.; Chupakhin, O. N. Green Chem. 2020, 22, 302.
doi: 10.1039/C9GC03414E |
| [23] |
Friscic, T.; Mottillo, C.; Titi, H. M. Angew. Chem., Int. Ed. 2020, 59, 1018.
doi: 10.1002/anie.v59.3 |
| [24] |
Li, Z.; Li, C.-J. J. Am. Chem. Soc. 2005, 127, 3672.
doi: 10.1021/ja050058j |
| [25] |
Meng, Q.-Y.; Liu, Q.; Zhong, J.-J.; Zhang, H.-H.; Li, Z.-J.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Org. Lett. 2012, 14, 5992.
doi: 10.1021/ol3028785 |
| [26] |
Pan, Y.; Kee, C. W.; Chen, L.; Tan, C.-H. Green Chem. 2011, 13, 2682.
doi: 10.1039/c1gc15489c |
| [27] |
Liu, Q.; Li, Y.-N.; Zhang, H.-H.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Chem.-Eur. J. 2012, 18, 620.
doi: 10.1002/chem.v18.2 |
| [28] |
Liu, W.; Su, Q.; Ju, P.; Guo, B.; Zhou, H.; Li, G.; Wu, Q. ChemSusChem 2017, 10, 664.
doi: 10.1002/cssc.201601702 |
| [29] |
Quan, Y.; Li, Q.-Y.; Zhang, Q.; Zhang, W.-Q.; Lu, H.; Yu, J.-H.; Chen, J.; Zhao, X.; Wang, X.-J. RSC Adv. 2016, 6, 23995.
doi: 10.1039/C6RA03516G |
| [30] |
Su, W. K.; Yu, J.; Li, Z.; Jiang, Z. J. Org. Chem. 2011, 76, 9144.
doi: 10.1021/jo2015533 |
| [31] |
Sud, A.; Sureshkumar, D.; Klussmann, M. Chem. Commun. 2009,3169.
|
| [32] |
Rueping, M.; Vila, C.; Koenigs, R. M.; Poscharny, K.; Fabry, D. C. Chem. Commun. 2011, 47, 2360.
doi: 10.1039/C0CC04539J |
| [33] |
Zhang, J.; Tiwari, B.; Xing, C.; Chen, X.; Chi, Y. B. Angew. Chem., Int. Ed. 2012, 51, 3649.
doi: 10.1002/anie.v51.15 |
| [34] |
Xie, Z.; Zan, X.; Sun, S.; Pan, X.; Liu, L. Org. Lett. 2016, 18, 3944.
doi: 10.1021/acs.orglett.6b01625 |
| [35] |
Zhang, G.; Ma, Y.; Wang, S.; Kong, W.; Wang, R. Chem. Sci. 2013, 4, 2645.
doi: 10.1039/c3sc50604e |
| [36] |
Yang, Q.; Zhang, L.; Ye, C.; Luo, S.; Wu, L.-Z.; Tung, C.-H. Angew. Chem., Int. Ed. 2017, 56, 3694.
doi: 10.1002/anie.201700572 |
| [37] |
Fu, N.; Li, L.; Yang, Q.; Luo, S. Org. Lett. 2017, 19, 2122.
doi: 10.1021/acs.orglett.7b00746 |
| [38] |
Li, Z.; Li, C.-J. Eur. J. Org. Chem. 2005, 2005, 3173.
doi: 10.1002/(ISSN)1099-0690 |
| [39] |
Zhang, Y.; Li, C.-J. Angew. Chem., Int. Ed. 2006, 45, 1949.
doi: 10.1002/(ISSN)1521-3773 |
| [40] |
Li, F.; Meng, Z.; Hua, J.; Li, W.; Lou, H.; Liu, L. Org. Biomol. Chem. 2015, 13, 5710.
doi: 10.1039/C5OB00277J |
| [41] |
Xiang, M.; Meng, Q.-Y.; Li, J.-X.; Zheng, Y.-W.; Ye, C.; Li, Z.-J.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Chem.-Eur. J. 2015, 21, 18080.
doi: 10.1002/chem.201503361 |
| [42] |
Zhang, G.; Zhang, Y.; Wang, R. Angew. Chem., Int. Ed. 2011, 50, 10429.
doi: 10.1002/anie.201105123 |
| [43] |
Gao, X.-W.; Meng, Q.-Y.; Xiang, M.; Chen, B.; Feng, K.; Tung, C.-H.; Wu, L.-Z. Adv. Synth. Catal. 2013, 355, 2158.
doi: 10.1002/adsc.201300311 |
| [44] |
Gao, X.-W.; Meng, Q.-Y.; Li, J.-X.; Zhong, J.-J.; Lei, T.; Li, X.-B.; Tung, C.-H.; Wu, L.-Z. ACS Catal. 2015, 5, 2391.
doi: 10.1021/acscatal.5b00093 |
| [45] |
Guo, C.; Song, J.; Luo, S.-W.; Gong, L.-Z. Angew. Chem., Int. Ed. 2010, 49, 5558.
doi: 10.1002/anie.201002108 |
| [46] |
Yu, J.; Ying, P.; Wang, H.; Xiang, K.; Su, W. Adv. Synth. Catal. 2020, 362, 893.
doi: 10.1002/adsc.v362.4 |
| [47] |
Shan, N.; Toda, F.; Jones, W. Chem. Commun. 2002,2372.
|
| [48] |
Yu, J.-B.; Zhang, Y.; Jiang, Z.-J.; Su, W.-K. J. Org. Chem. 2016, 81, 11514.
doi: 10.1021/acs.joc.6b02197 |
| [49] |
Benfatti, F.; Capdevila, M. G.; Zoli, L.; Benedetto, E.; Cozzi, P. G. Chem. Commun. 2009,5919.
|
| [50] |
Ho, X.-H.; Mho, S.-I.; Kang, H.; Jang, H.-Y. Eur. J. Org. Chem. 2010, 2010, 4436.
|
| [51] |
Larionov, E.; Mastandrea, M. M.; Pericas, M. A. ACS Catal. 2017, 7, 7008.
doi: 10.1021/acscatal.7b02659 |
| [52] |
Li, Z.; Li, C.-J. J. Am. Chem. Soc. 2005, 127, 6968.
doi: 10.1021/ja0516054 |
| [53] |
Alagiri, K.; Kumara, G. S. R.; Prabhu, R. Chem. Commun. 2011, 47, 11787.
doi: 10.1039/c1cc15050b |
| [54] |
Meng, Q.-Y.; Zhong, J.-J.; Liu, Q.; Gao, X.-W.; Zhang, H.-H.; Lei, T.; Li, Z.-J.; Feng, K.; Chen, B.; Tung, C.-H.; Wu, L.-Z. J. Am. Chem. Soc. 2013, 135, 19052.
doi: 10.1021/ja408486v |
| [55] |
Zhong, J.-J.; Meng, Q.-Y.; Liu, B.; Li, X.-B.; Gao, X.-W.; Lei, T.; Wu, C.-J.; Li, Z.-J.; Tung, C.-H.; Wu, L.-Z. Org. Lett. 2014, 16, 1988.
doi: 10.1021/ol500534w |
| [56] |
Zhong, J.-J.; Wu, C.-J.; Meng, Q.-Y.; Gao, X.-W.; Lei, T.; Tung, C.-H.; Wu, L.-Z. Adv. Synth. Catal. 2014, 356, 2846.
doi: 10.1002/adsc.201400588 |
| [57] |
Wu, C.-J.; Zhong, J.-J.; Meng, Q.-Y.; Lei, T.; Gao, X.-W.; Tung, C.-H.; Wu, L.-Z. Org. Lett. 2015, 17, 884.
doi: 10.1021/ol503744a |
| [58] |
Yang, Q.; Li, S.; Wang, J. Org. Chem. Front. 2018, 5, 577.
doi: 10.1039/C7QO00875A |
| [59] |
Kibriya, G.; Bagdi, A. K.; Hajra, A. J. Org. Chem. 2018, 83, 10619.
doi: 10.1021/acs.joc.8b01433 |
| [60] |
Maruoka, K.; Ooi, T. Chem. Rev. 2003, 103, 3013.
pmid: 12914490 |
| [61] |
Wu, J.-C.; Song, R.-J.; Wang, Z.-Q.; Huang, X.-C.; Xie, Y.-X.; Li, J.-H. Angew. Chem. 2012, 124, 3509.
doi: 10.1002/ange.v124.14 |
| [62] |
Huo, C.; Wang, C.; Wu, M.; Jia, X.; Xie, H.; Yuan, Y. Adv. Synth. Catal. 2014, 356, 411.
doi: 10.1002/adsc.201300535 |
| [63] |
Zhang, Y.; Ni, M.; Feng, B. Org. Biomol. Chem. 2016, 14, 1550.
doi: 10.1039/C5OB02325D |
| [64] |
Huo, C.; Yuan, Y.; Wu, M.; Jia, X.; Wang, X.; Chen, F.; Tang, J. Angew. Chem., Int. Ed. 2014, 53, 13544.
doi: 10.1002/anie.201406905 |
| [65] |
Huo, C.; Wang, C.; Sun, C.; Jia, X.; Wang, X.; Chang, W.; Wu, M. Adv. Synth. Catal. 2013, 355, 1911.
doi: 10.1002/adsc.v355.10 |
| [66] |
Zhu, S.; Rueping, M. Chem. Commun. 2012, 48, 11960.
doi: 10.1039/c2cc36995h |
| [67] |
Wang, Z.-Q.; Hu, M.; Huang, X.-C.; Gong, L.-B.; Xie, Y.-X. Li, J.-H. J. Org. Chem. 2012, 77, 8705.
doi: 10.1021/jo301691h |
| [68] |
Ni, C.; Chen, W.; Jiang, C.; Lu, H. New J. Chem. 2020, 44, 313.
doi: 10.1039/C9NJ05211A |
| [69] |
Li, K.; Tan, G.; Huang, J.; Song, F.; You, J. Angew. Chem., Int. Ed. 2013, 52, 12942.
doi: 10.1002/anie.201306181 |
| [70] |
Shen, M.-L.; Shen, Y.; Wang, P.-S. Org. Lett. 2019, 21, 2993.
doi: 10.1021/acs.orglett.9b00442 |
| [71] |
Xu, Z.; Yu, X.; Feng, X.; Bao, M. Beilstein J. Org. Chem. 2012, 8, 1564.
doi: 10.3762/bjoc.8.178 |
| [72] |
Salman, M.; Zhu, Z.-Q.; Huang, Z.-Z. Org. Lett. 2016, 18, 1526.
doi: 10.1021/acs.orglett.6b00162 |
| [73] |
Li, S.; Yang, X.; Wang, Y.; Zhou, H.; Zhang, B.; Huang, G.; Zhang, Y.; Li, Y. Adv. Synth. Catal. 2018, 360, 4452.
doi: 10.1002/adsc.v360.22 |
| [74] |
Zhang, Y.; Li, S.; Zhu, Y.; Yang, X.; Zhou, H.; Li, Y. J. Org. Chem. 2020, 85, 6261.
doi: 10.1021/acs.joc.9b01440 |
| [75] |
Enguehard-Gueiffier, C.; Gueiffier, A. Mini-Rev. Med. Chem. 2007, 7, 888.
pmid: 17897079 |
| [76] |
Jiao, J.; Zhang, J.-R.; Liao, Y.-Y.; Xu, L.; Hu, M.; Tang, R.-Y. RSC Adv. 2017, 7, 30152.
doi: 10.1039/C7RA05303G |
| [77] |
Zhu, Z.-Q.; Xiao, L.-J.; Zhou, C.-C.; Song, H.-L.; Xie, Z.-B.; Le, Z.-G. Tetrahedron Lett. 2018, 59, 3326.
doi: 10.1016/j.tetlet.2018.07.047 |
| [78] |
Ramana, D. V.; Chowhan, L. R.; Chandrasekharam, M. ChemistrySelect 2017, 2, 2241.
doi: 10.1002/slct.201700330 |
| [79] |
Sun, B.; Deng, J.; Li, D.; Jin, C.; Su, W. Tetrahedron Lett. 2018, 59, 4364.
doi: 10.1016/j.tetlet.2018.10.067 |
| [80] |
He, T.; Yu, L.; Zhang, L.; Wang, L.; Wang, M. Org. Lett. 2011, 13, 5016.
doi: 10.1021/ol201779n |
| [81] |
Xie, Z.; Cai, Y; Hu, H.; Lin, C.; Jiang, J.; Chen, Z.; Wang, L.; Pan, Y. Org. Lett. 2013, 15, 4600.
doi: 10.1021/ol4022113 |
| [82] |
Li, Y.; Wang, M.; Fan, W.; Qian, F.; Li, G.; Lu, H. J. Org. Chem. 2016, 81, 11743.
doi: 10.1021/acs.joc.6b02211 |
| [83] |
Dong, J.; Xia, Q.; Lv, X.; Yan, C.; Song, H.; Liu, Y.; Wang, Q. Org. Lett. 2018, 20, 5661.
doi: 10.1021/acs.orglett.8b02389 |
| [84] |
Singsardar, M.; Laru, S.; Mondal, S.; Hajra, A. J. Org. Chem. 2019, 84, 4543.
doi: 10.1021/acs.joc.9b00318 |
| [85] |
Ajani, O. O. Eur. J. Med. Chem. 2014, 85, 688.
doi: 10.1016/j.ejmech.2014.08.034 |
| [86] |
Yuan, J.; Fu, J.; Yin, J.; Dong, Z.; Xiao, Y.; Mao, P.; Qu, L. Org. Chem. Front. 2018, 5, 2820.
doi: 10.1039/C8QO00731D |
| [87] |
Wei, W.; Wang, L.; Yue, H.; Bao, P.; Liu, W.; Hu, C.; Yang, D.; Wang, H. ACS Sustainable Chem. Eng. 2018, 6, 17252.
doi: 10.1021/acssuschemeng.8b04652 |
| [88] |
Mane, K. D.; Kamble, R. B.; Suryavanshi, G. New J. Chem. 2019, 43, 7403.
doi: 10.1039/C9NJ00075E |
| [89] |
Li, Z.; Li, C.-J. Org. Lett. 2004, 6, 4997.
doi: 10.1021/ol047814v |
| [90] |
Sun, S.; Li, C.; Floreancig, P. E.; Lou, H.; Liu, L. Org. Lett. 2015, 17, 1684.
doi: 10.1021/acs.orglett.5b00447 |
| [91] |
Marset, X.; Perez J. M.; Ramon, D. J. Green Chem. 2016, 18, 826.
doi: 10.1039/C5GC01745A |
| [92] |
Huang, T.; Liu, X.; Lang, J.; Xu, J.; Li, L.; Feng, X. ACS Catal. 2017, 7, 5654.
doi: 10.1021/acscatal.7b01912 |
| [93] |
Yu, J.; Li, Z.; Jia, K.; Jiang, Z.; Liu, M.; Su, W. Tetrahedron Lett. 2013, 54, 2006.
doi: 10.1016/j.tetlet.2013.02.007 |
| [94] |
Perepichka, I.; Kundu, S.; Hearne, Z.; Li, C.-J. Org. Biomol. Chem. 2015, 13, 447.
doi: 10.1039/C4OB02138J |
| [95] |
Gaster, E.; Vainer, Y.; Regev, A.; Narute, S.; Sudheendran, K.; Werbeloff, A.; Shalit, H.; Pappo, D. Angew. Chem., Int. Ed. 2015, 54, 4198.
doi: 10.1002/anie.201409694 |
| [96] |
More, N. Y.; Jeganmohan, M. Org. Lett. 2015, 17, 3042.
doi: 10.1021/acs.orglett.5b01324 |
| [97] |
Morimoto, K.; Sakamoto, K.; Ohshika, T.; Dohi, T.; Kita, Y. Angew. Chem., Int. Ed. 2016, 55, 3652.
doi: 10.1002/anie.201511007 |
| [98] |
Dohi, T.; Kamitanaka, T.; Watanabe, S.; Hu, Y.; Washimi, N.; Kita, Y. Chem.-Eur. J. 2012, 18, 13614.
doi: 10.1002/chem.201202086 |
| [99] |
Morimoto, K.; Sakamoto, K.; Ohnishi, Y.; Miyamoto, T.; Ito, M.; Dohi, T.; Kita, Y. Chem.-Eur. J. 2013, 19, 8726.
doi: 10.1002/chem.v19.27 |
| [100] |
Dohi, T.; Ito, M.; Itani, I.; Yamaoka, N.; Morimoto, K.; Fujioka, H.; Kita, Y. Org. Lett. 2011, 13, 6208.
doi: 10.1021/ol202632h |
| [101] |
Kirste, A.; Schnakenburg, G.; Stecker, F.; Fischer, A.; Waldvogel, S. R. Angew. Chem., Int. Ed. 2010, 49, 971.
doi: 10.1002/anie.200904763 |
| [102] |
Kirste, A.; Schnakenburg, G.; Waldvogel, S. R. Org. Lett. 2011, 13, 3126.
doi: 10.1021/ol201030g |
| [103] |
Kirste, A.; Elsler, B.; Schnakenburg, G.; Waldvogel, S. R. J. Am. Chem. Soc. 2012, 134, 3571.
doi: 10.1021/ja211005g |
| [104] |
Elsler, B.; Schollmeyer, D.; Dyballa, K. M.; Franke, R.; Waldvogel, S. R. Angew. Chem., Int. Ed. 2014, 53, 5210.
|
| [105] |
Wiebe, A.; Schollmeyer, D.; Dyballa, K. M.; Franke, R.; Waldvogel, S. R. Angew. Chem., Int. Ed. 2016, 55, 11801.
doi: 10.1002/anie.201604321 |
| [106] |
Rockl, J. L.; Schollmeyer, D.; Franke, R.; Waldvogel, S. R. Angew. Chem., Int. Ed. 2020, 59, 315.
doi: 10.1002/anie.v59.1 |
| [107] |
Eisenhofer, A.; Hioe, J.; Gschwind, R. M.; König, B. Eur. J. Org. Chem. 2017, 2017, 2194.
doi: 10.1002/ejoc.201700211 |
| [108] |
Matsumoto, K.; Yoshida, M.; Shindo, M. Angew. Chem., Int. Ed. 2016, 55, 5272.
doi: 10.1002/anie.201600400 |
| [109] |
Matsumoto, K.; Takeda, S.; Hirokane, T.; Yoshida, M. Org. Lett. 2019, 21, 7279.
doi: 10.1021/acs.orglett.9b02527 |
| [110] |
Schulz, L.; Enders, M.; Elsler, B.; Schollmeyer, D.; Dyballa, K. M.; Franke, R.; Waldvogel, S. R. Angew. Chem., Int. Ed. 2017, 56, 4877.
doi: 10.1002/anie.201612613 |
| [111] |
Schulz, L.; Franke, R.; Waldvogel, S. R. ChemElectroChem 2018, 5, 2069.
doi: 10.1002/celc.v5.15 |
| [112] |
Luo, M.-J.; Li, Y.; Ouyang, X.-H.; Li, J.-H.; He, D.-L. Chem. Commun. 2020, 56, 2707.
doi: 10.1039/C9CC09879H |
| [113] |
Chandrasekharam, M.; Chiranjeevi, B.; Gupta, K. S. V.; Sridhar, B. J. Org. Chem. 2011, 76, 10229.
doi: 10.1021/jo202152b |
| [114] |
Akondi, A. M.; Trivedi, R.; Sreedhar, B.; Kantam, M. L.; Bhargava, S. Catal. Today 2012, 198, 35.
doi: 10.1016/j.cattod.2012.05.029 |
| [115] |
Wang, J.; Zhao, Y.; Gao, H.; Gao, G.-L.; Yang, C.; Xia, W. Asian J. Org. Chem. 2017, 6, 1402.
doi: 10.1002/ajoc.v6.10 |
| [116] |
Dahms, B.; Franke, R.; Waldvogel, S. R. ChemElectroChem 2018, 5, 1249.
doi: 10.1002/celc.v5.9 |
| [117] |
Guan, Z.-H.; Yan, Z.-Y.; Ren, Z.-H.; Liu, X.-Y.; Liang, Y.-M. Chem. Commun. 2010, 46, 2823.
doi: 10.1039/b923971e |
| [118] |
Wurtz, S.; Rakshit, S.; Neumann, J, J.; Droge, T.; Glorius, F. Angew. Chem., Int. Ed. 2008, 47, 7230.
doi: 10.1002/anie.v47:38 |
| [119] |
Bernini, R.; Fabrizi, G.; Sferrazza, A.; Cacchi, S. Angew. Chem., Int. Ed. 2009, 48, 8078.
doi: 10.1002/anie.v48:43 |
| [120] |
Neumann, J. J.; Rakshit, S.; Droge, T.; Wurtz, S.; Glorius, F. Chem.-Eur. J. 2011, 17, 7298.
doi: 10.1002/chem.v17.26 |
| [121] |
He, Z.; Liu, W.; Li, Z. Chem.-Asian J. 2011, 6, 1340.
doi: 10.1002/asia.v6.6 |
| [122] |
Jia, Z.; Nagano, T.; Li, X.; Chan, A. S. C. Eur. J. Org. Chem. 2013, 2013, 858.
doi: 10.1002/ejoc.201201585 |
| [123] |
Yu, W.; Du, Y.; Zhao, K. Org. Lett. 2009, 11, 2417.
doi: 10.1021/ol900576a |
| [124] |
Liu, J.; Wei, W.; Zhao, T.; Liu, X.; Wu, J.; Yu, W.; Chang, J. J. Org. Chem. 2016, 81, 9326.
doi: 10.1021/acs.joc.6b01960 |
| [125] |
Wu, C.-J. Meng, Q.-Y.; Lei, T.; Zhong, J.-J.; Liu, W.-Q.; Zhao, L.-M.; Li, Z.-J.; Chen, B.; Tung, C.-H.; Wu, L.-Z. ACS Catal. 2016, 6, 4635.
doi: 10.1021/acscatal.6b00917 |
| [126] |
Tang, S.; Gao, X.; Lei, A. Chem. Commun. 2017, 53, 3354.
doi: 10.1039/C7CC00410A |
| [127] |
Reux, B.; Nevalainen, T.; Raitio, K. H.; Koskinen, A. M. P. Bioorg. Med. Chem. 2009, 17, 4441.
doi: 10.1016/j.bmc.2009.05.013 |
| [128] |
Costa, E. V.; Pinheiro, M. L. B.; Barison, A.; Campos, F. R.; Salvador, M. J.; Maia, B. H. L. N. S.; Cabral, E. C.; Eberlin, M. N.; J. Nat. Prod. 2010, 73, 1180.
doi: 10.1021/np100013r |
| [129] |
Nishikawa-Shimono, R.; Sekiguchi, Y.; Koami, T.; Kawamura, M.; Wakasugi, D.; Watanabe, K.; Wakahara, S.; Kimura, K.; Yamanobe S.; Takayama, T. Bioorg. Med. Chem. 2013, 21, 7674.
doi: 10.1016/j.bmc.2013.10.025 |
| [130] |
Gao, F.; Liu, H.; Li, L.; Guo, J.; Wang, Y.; Zhao M.; Peng, S. Bioorg. Med. Chem. Lett. 2015, 25, 4434.
doi: 10.1016/j.bmcl.2015.09.014 |
| [131] |
Matcha, K.; Antonchick, A. P. Angew. Chem., Int. Ed. 2013, 52, 2082.
doi: 10.1002/anie.v52.7 |
| [132] |
Chen, J.; Wan, M.; Hua, J.; Sun, Y.; Lv, Z.; Li, W.; Liu, L. Org. Biomol. Chem. 2015, 13, 11561.
doi: 10.1039/C5OB01763G |
| [133] |
Zhang, L.; Zhang, G.; Li, Y.; Wang, S.; Lei, A. Chem. Commun. 2018, 54, 5744.
doi: 10.1039/C8CC02342E |
| [134] |
Obst, M.; König, B. Beilstein J. Org. Chem. 2016, 12, 2358.
doi: 10.3762/bjoc.12.229 |
| [135] |
Hernandez, J. G. Beilstein J. Org. Chem. 2017, 13, 1463.
doi: 10.3762/bjoc.13.144 |
| [136] |
Toda, F.; Tanaka, K.; Sekikawa, A. Chem. Commun. 1987,279.
|
| [137] |
Sokolov, A. N.; Bucar, D.-K.; Baltrusaitis, J.; Gu, S.X. MacGillivray, L. R. Angew. Chem., Int. Ed. 2010, 49, 4273.
doi: 10.1002/anie.v49:25 |
| [138] |
Stojakovic, J.; Farris, B. S.; MacGillivray, L. R. Faraday Discuss. 2014, 170, 35.
doi: 10.1039/C4FD00006D |
| [139] |
Strukil, V.; Sajko, I. Chem. Commun. 2017, 53, 9101.
doi: 10.1039/C7CC03510A |
| [140] |
Obst, M. König, B. Eur. J. Org. Chem. 2018, 2018, 4213.
doi: 10.1002/ejoc.201800556 |
| [141] |
Kubota, K.; Pang, Y.; Miura, A.; Ito, H. Science 2019, 366, 1500.
doi: 10.1126/science.aay8224 |
| [1] | Boyu Yan, Jieliang Wu, Jinfei Deng, Dan Chen, Xiushen Ye, Qiuli Yao. Recent Progress in Light-Driven Direct Dehydroxylation and Derivation of Alcohols [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3055-3066. |
| [2] | Zhongrong Xu, Jieping Wan, Yunyun Liu. Transition Metal-Free C—H Thiocyanation and Selenocyanation Based on Thermochemical, Photocatalytic and Electrochemical Process [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2425-2446. |
| [3] | Zhou Zhang, Yu Guo, Jing Yang, Dan Wu, Jiaxin Wang, Xinyue Hong, Peijun Cai, Liangce Rong. Electrochemically Promoted Halogenation of Imidazoland-[1,2-a]pyridine with Dichloro(bromo)ethylene and Iodoform [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2104-2109. |
| [4] | Linlin Du, Hua Zhang. Photochemical and Electrochemical Borylation Involving Aryl and Alkyl Compounds [J]. Chinese Journal of Organic Chemistry, 2023, 43(5): 1726-1741. |
| [5] | Wanjie Wei, Lei Zhan, Lei Gao, Guobao Huang, Xianli Ma. Research Progress of Electrochemical Synthesis of C-Sulfonyl Compounds [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 17-35. |
| [6] | Dongping Xu, Fei Huang, Lin Tang, Xinming Zhang, Wu Zhang. Visible Light-Induced Hydroxyalkylation of Heteroarenes with Aliphatic Alcohols [J]. Chinese Journal of Organic Chemistry, 2022, 42(5): 1493-1500. |
| [7] | Yu Zheng, Shencheng Qian, Pengcheng Xu, Binnan Zheng, Shenlin Huang. Electrochemical Oxidative Thiocyanosulfonylation of Aryl Acetylenes [J]. Chinese Journal of Organic Chemistry, 2022, 42(12): 4275-4281. |
| [8] | Xiuying Li, Pingfang Tao, Yongyu Cheng, Qiong Hu, Weijuan Huang, Yun Li, Zhihui Luo, Guobao Huang. Recent Progress on the Electrochemical Difunctionalization of Alkenes/Alkynes [J]. Chinese Journal of Organic Chemistry, 2022, 42(12): 4169-4201. |
| [9] | Hongxia Li, Peng Chen, Zhilin Wu, Yuhan Lu, Junmei Peng, Jingyang Chen, Weimin He. Electrochemical Oxidative Cross-Dehydrogenative Coupling of Five-Membered Aromatic Heterocycles with NH4SCN [J]. Chinese Journal of Organic Chemistry, 2022, 42(10): 3398-3404. |
| [10] | Yingjie Liu, Zhichuan Wang, Jianping Meng, Chen Li, Kai Sun. Research Progress of Photoelectric Co-catalysis [J]. Chinese Journal of Organic Chemistry, 2022, 42(1): 100-110. |
| [11] | Zhe Chang, Jiaxin Wang, Xi Lu, Yao Fu. Synthesis of gem-Difluoroalkenes through Nickel-Promoted Electrochemical Reductive Cross-Coupling [J]. Chinese Journal of Organic Chemistry, 2022, 42(1): 147-159. |
| [12] | Jintao Wu, Zhongquan Liu. Advances in Free-Radical Promoted C(sp3)—C(sp3) Bond Conversion [J]. Chinese Journal of Organic Chemistry, 2022, 42(1): 16-32. |
| [13] | Xiang Liu, Wen Li, Canzhan Zhuang, Hua Cao. Application of Photochemical/Electrochemical Synthesis in C—H Functionalization of Quinoxalin-2(1H)-one [J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3459-3481. |
| [14] | Junqing Gao, Xinjun Weng, Cong Ma, Xuetao Xu, Ping Fang, Tiansheng Mei. Electrochemical 2,2,6,6-tetramethylpiperidinyl-N-oxyl (TEMPO)-Mediated α-Cyanation and Phosphonylation of Cyclic Amines with Metal-Free Conditions [J]. Chinese Journal of Organic Chemistry, 2021, 41(8): 3223-3234. |
| [15] | Wei Meng, Kun Xu, Bingbing Guo, Chengchu Zeng. Recent Advances in Minisci Reactions under Electrochemical Conditions [J]. Chinese Journal of Organic Chemistry, 2021, 41(7): 2621-2635. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||