Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (8): 3097-3105.DOI: 10.6023/cjoc202104041 Previous Articles Next Articles
ARTICLES
于丽杰a, 冯勃b,c, 王智佳a, 高立信a,b, 张纯a,d, Rajendran Satheeshkumara, 李佳b, 周宇波b,c,*( ), 王文龙a,*(
), 王文龙a,*( )
)
收稿日期:2021-04-18
修回日期:2021-05-19
发布日期:2021-06-02
通讯作者:
周宇波, 王文龙
作者简介:基金资助:
Lijie Yua, Bo Fengb,c, Zhijia Wanga, Lixin Gaoa,b, Chun Zhanga,d, Rajendran Satheeshkumara, Jia Lib, Yubo Zhoub,c( ), Wenlong Wanga(
), Wenlong Wanga( )
)
Received:2021-04-18
Revised:2021-05-19
Published:2021-06-02
Contact:
Yubo Zhou, Wenlong Wang
About author:Supported by:Share
Lijie Yu, Bo Feng, Zhijia Wang, Lixin Gao, Chun Zhang, Rajendran Satheeshkumar, Jia Li, Yubo Zhou, Wenlong Wang. Synthesis of 5-Phenyl-1,3,4-thiadiazole Derivatives and Their Biochemical Evaluation against Src Homology 2 Domain-Containing Protein Tyrosine Phosphatase 1 (SHP1)[J]. Chinese Journal of Organic Chemistry, 2021, 41(8): 3097-3105.
| Compd. | R1 | R2 | R3 | SHP1 | |
|---|---|---|---|---|---|
| Inhibition/% at 50 μmol/L | IC50/(μmol•L–1) | ||||
| 2a | 3-NO2 | 4-NHMe | NH2 | 27.5±19.1 | — |
| 2b | 3-NO2 | | NH2 | 20.9±16.9 | — |
| 2c | 3-NO2 | 4-OMe | NH2 | 21.6±5.1 | — |
| 2d | 3-OMe | 4-OMe | NH2 | –2.4±7.2 | — |
| 2e | 3-OH | 3-OH | NH2 | 8.6±1.8 | — |
| 4 | 3-NO2 | 4-NHMe | | 80.6±6.5 | 23.42±3.17 |
| 5a | 3-NO2 | 4-NHMe | | 64.3±11.0 | 4.40±1.09 |
| 5b | 3-NO2 | 4-NHMe | | 92.8±2.1 | 1.33±0.16 |
| 5c | 3-NO2 | 4-NHMe | | 69.7±2.7 | 21.56±2.61 |
| 5d | 3-NO2 | | | 36.4±8.4 | — |
| 5e | 3-NO2 | 4-OMe | | 38.6±39.4 | — |
| NSC-87877 | — | — | — | — | 34.76±4.46 |
| Compd. | R1 | R2 | R3 | SHP1 | |
|---|---|---|---|---|---|
| Inhibition/% at 50 μmol/L | IC50/(μmol•L–1) | ||||
| 2a | 3-NO2 | 4-NHMe | NH2 | 27.5±19.1 | — |
| 2b | 3-NO2 | | NH2 | 20.9±16.9 | — |
| 2c | 3-NO2 | 4-OMe | NH2 | 21.6±5.1 | — |
| 2d | 3-OMe | 4-OMe | NH2 | –2.4±7.2 | — |
| 2e | 3-OH | 3-OH | NH2 | 8.6±1.8 | — |
| 4 | 3-NO2 | 4-NHMe | | 80.6±6.5 | 23.42±3.17 |
| 5a | 3-NO2 | 4-NHMe | | 64.3±11.0 | 4.40±1.09 |
| 5b | 3-NO2 | 4-NHMe | | 92.8±2.1 | 1.33±0.16 |
| 5c | 3-NO2 | 4-NHMe | | 69.7±2.7 | 21.56±2.61 |
| 5d | 3-NO2 | | | 36.4±8.4 | — |
| 5e | 3-NO2 | 4-OMe | | 38.6±39.4 | — |
| NSC-87877 | — | — | — | — | 34.76±4.46 |

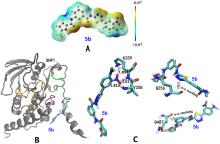
| Compd. | SHP1 | SHP2 | SHP2/SHP1 | PTP1B | TCPTP |
|---|---|---|---|---|---|
| 5b | 1.33±0.16 | 2.99±0.36 | 2.25 | NAb | NAb |
| NSC-87877 | 34.76±4.46 | 33.71±0.72 | 50.04±11.91 | 40.70±6.50 |
| Compd. | SHP1 | SHP2 | SHP2/SHP1 | PTP1B | TCPTP |
|---|---|---|---|---|---|
| 5b | 1.33±0.16 | 2.99±0.36 | 2.25 | NAb | NAb |
| NSC-87877 | 34.76±4.46 | 33.71±0.72 | 50.04±11.91 | 40.70±6.50 |



| [1] |
Tonks, N. K. FEBS J. 2013, 280, 346.
|
| [2] |
Hendriks, W.; Elson, A.; Harroch, S.; Pulido, R.; Stoker, A.; Hertog, J. D. FEBS J. 2013, 280, 708.
doi: 10.1111/febs.12000 pmid: 22938156 |
| [3] |
Frankson, R.; Yu, Z. H.; Bai, Y.; Li, Q.; Zhang, R. Y.; Zhang, Z. Y. Cancer Res. 2017, 77, 5701.
doi: 10.1158/0008-5472.CAN-17-1510 |
| [4] |
Varone, A.; Spano, D.; Corda, D. Front. Oncol. 2020, 10, 935.
doi: 10.3389/fonc.2020.00935 |
| [5] |
Sandur, S. K.; Pandey, M. K.; Sung, B.; Aggarwal, B. B. Mol. Cancer Res. 2010, 8, 107.
doi: 10.1158/1541-7786.MCR-09-0257 |
| [6] |
Sharma, Y.; Bashir, S.; Bhardwaj, P.; Ahmad, A.; Khan, F. Immunol. Res. 2016, 64, 804.
doi: 10.1007/s12026-016-8805-y pmid: 27216862 |
| [7] |
Yuan, X.; Bu, H.; Zhou, J.; Yang, C. Y.; Zhang, H. J. Med. Chem. 2020, 63, 11368
doi: 10.1021/acs.jmedchem.0c00249 |
| [8] |
Dempke, W. M.; Peter, U.; Klaus, F.; Timothy, C. Oncology 2018, 95, 1.
|
| [9] |
Song, M.; Park, J. E.; Park, S. G.; Lee, D. H.; Choi, H. K.; Park, B. C. Biochem. Biophys. Res. Commun. 2009, 381, 491.
doi: 10.1016/j.bbrc.2009.02.069 |
| [10] |
Kundu, S.; Fan, K.; Cao, M.; Lindner, D. J.; Zhao, Z. J.; Borden, E.; Yi, T. L. J. Immunol. 2010, 184, 6529.
doi: 10.4049/jimmunol.0903562 |
| [11] |
Arabaci, G.; Yi, T.; Fu, H.; Porter, M. E.; Beebe, K. D.; Pei, D. Bioorg. Med. Chem. Lett. 2002, 12, 3047.
pmid: 12372498 |
| [12] |
Yi, T.; Elson, P.; Mitsuhashi, M.; Jacobs, B.; Hollovary, E.; Budd, G. T.; Spiro, T.; Triozzi, E.; Borden, E. C. Oncotarget 2011, 2, 1155.
doi: 10.18632/oncotarget.v2i12 |
| [13] |
Lu, L.; Wang, S.; Zhu, M.; Liu, Z.; Guo, M.; Xing, S.; Fu, X. Biometals 2010, 23, 1139.
doi: 10.1007/s10534-010-9363-8 |
| [14] |
Wang, Q.; Zhu, M.; Lu, L.; Yuan, C.; Fu, X. Dalton. Trans. 2011, 40, 12926.
doi: 10.1039/c1dt11006c |
| [15] |
Akiba, H.; Sumaoka, J.; Hamakubo, T.; Komiyama, M. Anal. Bioanal. Chem. 2014, 406, 2957.
doi: 10.1007/s00216-014-7707-x |
| [16] |
Frija, L.; Pombeiro, A.; Kopylovich, M. N. Eur. J. Org. Chem. 2017, 19, 2670.
|
| [17] |
Yang, H.; Li, C. Y.; Wang, X. M.; Yang, Y. H.; Zhu, H. L. Chem. Rev. 2014. 45, 5572.
|
| [18] |
Wang, W. L.; Yang, D. L.; Gao, L. X.; Tang, C. L.; Ma, W. P.; Ye, H. H.; Zhang, S. Q.; Zhao, Y. N.; Xu, H. J.; Hu, Z.; Chen, X.; Fan, W. H.; Chen, H. J.; Li, J. Y.; Nan, F. J.; Li, J.; Feng, B. N. Molecules 2013, 19, 102.
doi: 10.3390/molecules19010102 |
| [19] |
Wang, W.L; Chen, X.; Gao, L.X; Sheng, L.; Li, J. Y.; Li, J.; Feng, B. N. Chem. Biol. Drug Des. 2015, 86, 1161.
doi: 10.1111/cbdd.12587 |
| [20] |
Satheeshkumar, R.; Zhu, R.; Feng, B.; Huang, C.; Gao, Y.; Gao, L. X.; Shen, C.; Hou, T. J.; Xu, L.; Li, J.; Zhu, Y. L.; Zhou, Y. B.; Wang, W. L. Bioorg. Med. Chem. Lett. 2020, 30, 127170.
doi: S0960-894X(20)30267-5 pmid: 32273218 |
| [21] |
Wang, W. L.; Chen, X. Y.; Gao, Y.; Gao, L. X.; Sheng, L.; Zhu, J. Y.; Xu, L.; Ding, Z. Z.; Zhang, C.; Li, J. Y.; Li, J.; Zhou, Y. B. Bioorg. Med. Chem. Lett. 2017, 27, 5154.
doi: 10.1016/j.bmcl.2017.10.059 |
| [22] |
Wang, W. L.; Huang, C.; Gao, L. X.; Tang, C. L.; Wang, J. Q.; Wu, M. C.; Sheng, L.; Chen, H. J.; Nan, F. J.; Li, J. Y.; Feng, B. N. Bioorg. Med. Chem. Lett. 2014, 24, 1889.
|
| [23] |
Wang, W. L.; Luo, H.; Gao, Y.; Gao, L. X.; Sheng, L.; Zhou, Y. B.; Li, J. Y.; Li, J.; Feng, B. N. Chin. J. Org. Chem. 2016, 36, 2142. (in Chinese)
doi: 10.6023/cjoc201603045 |
|
(王文龙, 骆欢, 高雅, 高立信, 盛丽, 周宇波, 李佳, 李静雅, 冯柏年, 有机化学, 2016, 36, 2142.)
doi: 10.6023/cjoc201603045 |
|
| [24] |
Pac, A.; As, A.; Fal, A.; Mb, B.; Mfe, C. J. Mol. Struct. 2019, 1179, 11.
doi: 10.1016/j.molstruc.2018.10.082 |
| [25] |
Chen, Y. N.; LaMarche, M. J.; Chan, H. M.; Fekkes, P.; Garcia, F. J.; Acker, M. G.; Antonakos, B.; Chen, C. H.; Chen, Z.; Cooke, V. G.; Dobson, J. R.; Deng, Z.; Fei, F.; Firestone, B.; Fodor, M.; Fridrich, C.; Gao, H.; Grunenfelder, D.; Hao, H. X.; Jacob, J.; Ho, S.; Hsiao, K.; Kang, Z. B.; Karki, R.; Kato, M.; Larrow, J.; LaBonte, L. R.; Lenoir, F.; Liu, G.; Liu, S.; Majumdar, D.; Meyer, M. J.; Palermo, M.; Perez, L.; Pu, M.; Price, E.; Quinn, C.; Shakya, S.; Shultz, M. D.; Slisz, J.; Venkatesan, K.; Wang, P.; Warmuth, M.; Williams, S.; Yang, G.; Yuan, J.; Zhang, J. H.; Zhu, P.; Ramsey, T.; Keen, N. J.; Sellers, W. R.; Stams, T.; Fortin, P. D. Nature 2016, 535, 148.
doi: 10.1038/nature18621 |
| [26] |
Welte, S.; Baringhaus, K. H.; Schmider, W.; Müller, G.; Petry, S.; Tennagels, N. Anal. Biochem. 2005, 338, 32.
doi: 10.1016/j.ab.2004.11.047 |
| [27] |
Krishnan, N.; Koveal, D.; Miller, D. H.; Xue, B.; Akshinthala, S. D.; Kragelj, J.; Jensen, M. R.; Gauss, C. M.; Page, R.; Blackledge, M.; Muthuswamy, S. K.; Peti, W.; Tonks, N. K. Nat. Chem. Biol. 2014, 10, 558.
doi: 10.1038/nchembio.1528 |
| [28] |
Zhang, W.; Hong, D.; Zhou, Y.; Zhang, Y.; Shen, Q.; Li, J. Y.; Hu, L. H.; Li, J. Biochim. Biophys. Acta 2006, 1760, 1505.
pmid: 16828971 |
| [29] |
McGovern, S. L.; Helfand, B. T.; Feng, B.; Shoichet, B. K. J. Med. Chem. 2003, 46, 4265.
pmid: 13678405 |
| [30] |
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A., Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Keith, T.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, O.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford, CT, 2013.
|
| [31] |
Morris, G. M.; Huey, R.; Lindstrom, W.; Sanner, M. F.; Belew, R. K.; Goodsell, D. S.; Olson, A. J. J. Comput. Chem. 2009, 30, 2785.
doi: 10.1002/jcc.v30:16 |
| [32] |
Yang, J.; Cheng, Z. L.; Niu, T. Q.; Liang, X. S.; Zhao, Z. Z. J.; Zhou, G. W. J. Biol. Chem. 2000, 275, 4066.
pmid: 10660565 |
| [33] |
Zhang, B.; Wang, K. B.; Wang, W.; Wang, X.; Liu, F.; Zhu, J. P.; Shi, J.; Li, L. Y.; Han, H.; Xu, K.; Qiao, H. Y.; Zhang, X.; Jiao, R. H.; Houk, K. N.; Liang, Y.; Tan, R. X.; Ge, H. M. Nature 2019, 568, 122.
doi: 10.1038/s41586-019-1021-x |
| [34] |
Daina, A.; Michielin, O.; Zoete, V. Sci. Rep. 2017, 7, 1.
doi: 10.1038/s41598-016-0028-x |
| [35] |
Vachala, S. D.; Bhargavi, B. J. Chem. Pharm. Res. 2014, 6, 377.
|
| [36] |
Guan, P.; Wan, Y. C.; Hou, X. B.; Wang, L; Xu, W. F.; Tang, W. P.; Fang, H. Bioorg. Med. Chem. 2014, 22, 5766.
|
| [37] |
Jakovljević, K.; Matić, I. Z.; Stanojković, T.; Krivokuća, A.; Marković, V.; Joksović, M. D.; Mihailović, N.; Nićiforović, M.; Joksović, L. Bioorg. Med. Chem. Lett. 2017, 27, 3709.
doi: S0960-894X(17)30696-0 pmid: 28709826 |
| [1] | Haibo Huo, Guixia Li, Shijun Wang, Chun Han, Baojun Shi, Jian Li. Novel γ-Carboline Derivatives as Antibacterial Agents: Synthesis and Antibacterial Evaluation [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 204-215. |
| [2] | Xingzhou Liu, Mingjia Yu, Jianhua Liang. Research Progress on the Synthesis of Protoberberine Skeleton and Its Anti-inflammatory Activity [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1325-1340. |
| [3] | Jianfei Gao, Shunyi Li, Yulong He, Yingxia Li, Heyao Wang, Erfang Huang, Chun Hu. Design, Synthesis and Biological Evaluation of FABP4/5 Inhibitors Based on Quinoline Scaffold [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 636-645. |
| [4] | Guangping Liang, Wei Wang, Xuxiu Zhu, Guangyan Liang, Jun Yang, Daoping Wang. Synthesis and in Vitro Anti-tumor Activity of Novel Spliced Compounds of Zidovudine and 4-Anilinoquinazolines [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2793-2805. |
| [5] | Rong Zhang, Xiang Gao, Lingling Chen, Fajun Nan. Discovery and Structure-Activity Relationship Studies of Thiazole- Oxazole Tandem Heterocyclic RNA Splicing Inhibitors [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2925-2939. |
| [6] | Ming Cai, Liang Shao, Fan Yang, Jihong Zhang, Fei Yu. Design, Synthesis of Pentacyclic Triterpenoid Glucose Conjugate and in vitro Activity against Influenza Virus [J]. Chinese Journal of Organic Chemistry, 2022, 42(5): 1453-1462. |
| [7] | Siyu Zhu, Xinyu Huo, Qin Ma, Wei Chen, Jie Zhang, Liang Guo. Design, Synthesis, and Antitumor Activity of β-Carboline-Benzimidazole Hybrids [J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 1129-1135. |
| [8] | Yan Zeng, Lifei Nie, Chao Niu, Aytilla Mamatjan, Khurshed Bozorov, Jiangyu Zhao, Haji Akber Aisa. Synthesis and Biological Activities of Dihydrooxazolo[5,4-d]-pyrrolo[1,2-a]pyrimidinones [J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 543-556. |
| [9] | Wei Wang, Furan Wu, Yidan Ma, Dan Xu, Gong Xu. Study on Synthesis and Antifungal Activity of Novel Benzamides Containing Substituted Pyrazole Unit [J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 607-618. |
| [10] | Fengxing Li, Xin Lu, Xu Liu, Lulu Su, Xiaoliu Li, Hua Chen. Structural Modification of Benzimidazole-Iminosugars and Their Inhibitory Activities against β-Glycosidases [J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3643-3651. |
| [11] | Zheng Zhang, Chengqiu Dai, Honghong Wu, Jingya Li, Fajun Nan. Design and Synthesis of Alkyl Phenols Inhibitors of Death Associated Apoptotic Protein Kinase 2 (DRAK2) [J]. Chinese Journal of Organic Chemistry, 2021, 41(8): 3204-3213. |
| [12] | Xu Liu, Lulu Su, Zhaoxi Zhou, Liping Niu, Ligang Gao, Huanhuan Ju, Fengxing Li, Xiaoliu Li, Hua Chen. Design and Synthesis of Benzimidazole-Iminosugars and Their Inhibitory Activities against Glycosidases [J]. Chinese Journal of Organic Chemistry, 2021, 41(7): 2861-2874. |
| [13] | Runqiu Lü, Wei Zhang, Lifang Yu. Recent Advances in Antitubercular Compounds Targeting Mycolic Acid Biosynthesis and Transport [J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2249-2260. |
| [14] | Liang Shao, Fan Yang, Weijia Li, Fei Yu. Design, Synthesis and Anti-influenza A Virus Evaluation of Oleanolic Acid C3-Glycoconjugates [J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2454-2466. |
| [15] | Zhang Qiying, Zhang Yiming, Hao Erjun, Bai Juan, Qu Guirong, Guo Haiming. Asymmetric Transfer Hydrogenation via Dynamic Kinetic Resolution for the Construction of Carbocyclic N3-Purine Nucleosides [J]. Chinese Journal of Organic Chemistry, 2020, 40(2): 376-383. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||