Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (1): 277-292.DOI: 10.6023/cjoc202106053 Previous Articles Next Articles
ARTICLES
陈睿嘉a, 周聪a, 逄锡文a, 刘佳君b, 顾玉诚c, 刘建文b, 李忠a,*( )
)
收稿日期:2021-06-29
修回日期:2021-08-02
发布日期:2021-09-02
通讯作者:
李忠
基金资助:
Ruijia Chena, Cong Zhoua, Xiwen Panga, Jiajun Liub, Yucheng Guc, Jianwen Liub, Zhong Lia( )
)
Received:2021-06-29
Revised:2021-08-02
Published:2021-09-02
Contact:
Zhong Li
Supported by:Share
Ruijia Chen, Cong Zhou, Xiwen Pang, Jiajun Liu, Yucheng Gu, Jianwen Liu, Zhong Li. Design, Synthesis, Anti-cancer Activities and Computational Analysis of Novel Diamides Conformationally Restricted by Cyclopropane[J]. Chinese Journal of Organic Chemistry, 2022, 42(1): 277-292.
| Compd. | Structure (Relative stereochemistry) | Growth-inhibition (200 μg•mL–1) | |||
|---|---|---|---|---|---|
| MCF-7 | BGC-823 | HepG2 | NCI-H460 | ||
| 1-2 | | 83.46±3.64 | 54.78±1.16 | 48.13±1.69 | 97.31±0.18 |
| 1-31 | | 19.42±1.19 | –28.92±2.03 | 38.24±5.37 | 14.20±6.58 |
| Compd. | Structure (Relative stereochemistry) | Growth-inhibition (200 μg•mL–1) | |||
|---|---|---|---|---|---|
| MCF-7 | BGC-823 | HepG2 | NCI-H460 | ||
| 1-2 | | 83.46±3.64 | 54.78±1.16 | 48.13±1.69 | 97.31±0.18 |
| 1-31 | | 19.42±1.19 | –28.92±2.03 | 38.24±5.37 | 14.20±6.58 |
| Compd. | R1 | Growth-inhibition (200 μg•mL–1) | |||
|---|---|---|---|---|---|
| MCF-7 | BGC-823 | HepG2 | NCI-H460 | ||
| 1-1 | Methyl | 37.23±3.34 | 32.39±1.17 | 23.47±4.79 | 47.37±0.50 |
| 1-2 | Ethyl | 83.46±3.64 | 54.78±1.16 | 48.13±1.69 | 97.31±0.18 |
| 1-3 | tert-Butyl | 85.73±2.92 | 4.17±0.52 | 57.57±1.57 | 94.34±2.25 |
| 1-4 | Benzyl | 96.83±0.69 | 97.75±0.08 | 99.14±0.28 | 98.42±0.16 |
| 1-30 | Phenethyl | 90.96±0.28 | 91.19±0.99 | 93.00±8.44 | 97.46±0.16 |
| Compd. | R1 | Growth-inhibition (200 μg•mL–1) | |||
|---|---|---|---|---|---|
| MCF-7 | BGC-823 | HepG2 | NCI-H460 | ||
| 1-1 | Methyl | 37.23±3.34 | 32.39±1.17 | 23.47±4.79 | 47.37±0.50 |
| 1-2 | Ethyl | 83.46±3.64 | 54.78±1.16 | 48.13±1.69 | 97.31±0.18 |
| 1-3 | tert-Butyl | 85.73±2.92 | 4.17±0.52 | 57.57±1.57 | 94.34±2.25 |
| 1-4 | Benzyl | 96.83±0.69 | 97.75±0.08 | 99.14±0.28 | 98.42±0.16 |
| 1-30 | Phenethyl | 90.96±0.28 | 91.19±0.99 | 93.00±8.44 | 97.46±0.16 |
| Compd. | R1 | IC50/(μg•mL–1) | |||
|---|---|---|---|---|---|
| MCF-7 | BGC-823 | HepG2 | NCI-H460 | ||
| 1-1 | Methyl | >200 | >200 | >200 | >200 |
| 1-2 | Ethyl | 33.81±2.06 | >200 | >200 | 29.80±2.91 |
| 1-3 | tert-Butyl | 29.27±5.83 | >200 | >200 | 38.38±1.40 |
| 1-4 | Benzyl | 40.79±2.18 | 50.90±0.99 | 26.09±2.97 | 26.78±2.78 |
| 1-5 | 4-Methylbenzyl | 32.29±1.7 | 21.25±3.03 | 10.44±1.60 | 20.38±1.21 |
| 1-6 | 3-Methylbenzyl | 40.35±5.19 | 35.50±3.97 | 35.01±1.54 | 30.59±1.18 |
| 1-7 | 2-Methylbenzyl | 33.80±1.72 | 25.42±2.29 | 23.20±1.07 | 21.60±1.60 |
| 1-8 | 4-Chlorobenzyl | 36.89±2.29 | 19.47±5.37 | 17.65±1.24 | 10.06±1.82 |
| 1-9 | 3-Chlorobenzyl | 36.80±4.39 | 30.58±2.65 | 28.62±0.81 | 22.48±0.71 |
| 1-10 | 2-Chlorobenzyl | 80.47±6.93 | 31.75±4.74 | 22.75±0.84 | 21.61±1.30 |
| 1-11 | 4-Fluorobenzyl | 67.45±2.43 | 29.55±2.70 | 27.07±1.05 | 25.27±2.85 |
| 1-12 | 4-Bromobenzyl | 17.65±0.99 | 28.29±2.54 | 21.17±0.81 | 21.01±1.25 |
| 1-13 | 4-Iodobenzyl | 27.57±6.26 | 26.24±2.70 | 26.29±1.43 | 26.15±1.36 |
| 1-14 | 4-Methoxybenzyl | 20.78±1.38 | 24.51±2.57 | 24.14±1.23 | 25.17±1.84 |
| 1-15 | 4-Difluoromethoxybenzyl | 75.61±14.80 | 50.03±2.97 | 39.52±6.27 | 56.80±10.58 |
| 1-16 | 4-tert-Butylbenzyl | >200 | 92.61±16.39 | >200 | 95.35±17.62 |
| 1-17 | 4-Cyanobenzyl | 47.75±9.06 | 42.49±4.12 | 37.92±2.94 | 30.26±3.15 |
| 1-18 | 4-Trifluoromethylbenzyl | 25.66±1.11 | 21.39±1.11 | 21.23±1.99 | 15.12±1.43 |
| 1-19 | (4-Phenoxyphenyl)methyl | 8.38±0.67 | 22.67±0.80 | 19.30±1.10 | 20.49±1.32 |
| 1-20 | 4-Phenylbenzyl | 25.88±2.95 | 24.97±1.27 | 27.06±3.07 | 22.94±1.15 |
| 1-21 | 2,4-Difluorobenzyl | 40.84±1.24 | 51.29±1.09 | 23.52±2.50 | 28.35±2.28 |
| 1-22 | 2,6-Dichlorobenzyl | >200 | >200 | 19.74±0.79 | 39.89±9.27 |
| 1-23 | 3,4,5-Trimethoxybenzyl | 19.16±3.01 | 34.39±3.52 | 27.22±2.61 | 20.39±1.41 |
| 1-24 | (Tetrahydrofuran-3-yl)methyl | 153.9±20.2 | 50.30±6.65 | 46.88±5.39 | 56.51±11.21 |
| 1-25 | (6-Chloropyridine-3-yl)methyl | 27.12±0.92 | 32.39±3.46 | 34.15±0.94 | 29.36±1.79 |
| 1-26 | (2-Chlorothiazole-5-yl)methyl | >200 | >200 | >200 | >200 |
| 1-27 | Furan-2-ylmethyl | 25.8±1.32 | 32.39±4.40 | 15.42±4.40 | 27.03±2.49 |
| 1-28 | Thiophene-3-ylmethyl | 83.43±4.37 | >200 | >200 | >200 |
| 1-29 | Naphthalene-1-ylmethyl | 32.53±2.82 | 22.91±1.20 | 23.55±1.97 | 20.52±0.98 |
| 1-30 | Phenethyl | 59.05±3.60 | 53.12±1.16 | 26.76±1.18 | 30.37±2.36 |
| 5-Fu | — | 8.23±1.33 | 10.31±1.40 | 7.09±1.61 | 2.26±0.80 |
| Compd. | R1 | IC50/(μg•mL–1) | |||
|---|---|---|---|---|---|
| MCF-7 | BGC-823 | HepG2 | NCI-H460 | ||
| 1-1 | Methyl | >200 | >200 | >200 | >200 |
| 1-2 | Ethyl | 33.81±2.06 | >200 | >200 | 29.80±2.91 |
| 1-3 | tert-Butyl | 29.27±5.83 | >200 | >200 | 38.38±1.40 |
| 1-4 | Benzyl | 40.79±2.18 | 50.90±0.99 | 26.09±2.97 | 26.78±2.78 |
| 1-5 | 4-Methylbenzyl | 32.29±1.7 | 21.25±3.03 | 10.44±1.60 | 20.38±1.21 |
| 1-6 | 3-Methylbenzyl | 40.35±5.19 | 35.50±3.97 | 35.01±1.54 | 30.59±1.18 |
| 1-7 | 2-Methylbenzyl | 33.80±1.72 | 25.42±2.29 | 23.20±1.07 | 21.60±1.60 |
| 1-8 | 4-Chlorobenzyl | 36.89±2.29 | 19.47±5.37 | 17.65±1.24 | 10.06±1.82 |
| 1-9 | 3-Chlorobenzyl | 36.80±4.39 | 30.58±2.65 | 28.62±0.81 | 22.48±0.71 |
| 1-10 | 2-Chlorobenzyl | 80.47±6.93 | 31.75±4.74 | 22.75±0.84 | 21.61±1.30 |
| 1-11 | 4-Fluorobenzyl | 67.45±2.43 | 29.55±2.70 | 27.07±1.05 | 25.27±2.85 |
| 1-12 | 4-Bromobenzyl | 17.65±0.99 | 28.29±2.54 | 21.17±0.81 | 21.01±1.25 |
| 1-13 | 4-Iodobenzyl | 27.57±6.26 | 26.24±2.70 | 26.29±1.43 | 26.15±1.36 |
| 1-14 | 4-Methoxybenzyl | 20.78±1.38 | 24.51±2.57 | 24.14±1.23 | 25.17±1.84 |
| 1-15 | 4-Difluoromethoxybenzyl | 75.61±14.80 | 50.03±2.97 | 39.52±6.27 | 56.80±10.58 |
| 1-16 | 4-tert-Butylbenzyl | >200 | 92.61±16.39 | >200 | 95.35±17.62 |
| 1-17 | 4-Cyanobenzyl | 47.75±9.06 | 42.49±4.12 | 37.92±2.94 | 30.26±3.15 |
| 1-18 | 4-Trifluoromethylbenzyl | 25.66±1.11 | 21.39±1.11 | 21.23±1.99 | 15.12±1.43 |
| 1-19 | (4-Phenoxyphenyl)methyl | 8.38±0.67 | 22.67±0.80 | 19.30±1.10 | 20.49±1.32 |
| 1-20 | 4-Phenylbenzyl | 25.88±2.95 | 24.97±1.27 | 27.06±3.07 | 22.94±1.15 |
| 1-21 | 2,4-Difluorobenzyl | 40.84±1.24 | 51.29±1.09 | 23.52±2.50 | 28.35±2.28 |
| 1-22 | 2,6-Dichlorobenzyl | >200 | >200 | 19.74±0.79 | 39.89±9.27 |
| 1-23 | 3,4,5-Trimethoxybenzyl | 19.16±3.01 | 34.39±3.52 | 27.22±2.61 | 20.39±1.41 |
| 1-24 | (Tetrahydrofuran-3-yl)methyl | 153.9±20.2 | 50.30±6.65 | 46.88±5.39 | 56.51±11.21 |
| 1-25 | (6-Chloropyridine-3-yl)methyl | 27.12±0.92 | 32.39±3.46 | 34.15±0.94 | 29.36±1.79 |
| 1-26 | (2-Chlorothiazole-5-yl)methyl | >200 | >200 | >200 | >200 |
| 1-27 | Furan-2-ylmethyl | 25.8±1.32 | 32.39±4.40 | 15.42±4.40 | 27.03±2.49 |
| 1-28 | Thiophene-3-ylmethyl | 83.43±4.37 | >200 | >200 | >200 |
| 1-29 | Naphthalene-1-ylmethyl | 32.53±2.82 | 22.91±1.20 | 23.55±1.97 | 20.52±0.98 |
| 1-30 | Phenethyl | 59.05±3.60 | 53.12±1.16 | 26.76±1.18 | 30.37±2.36 |
| 5-Fu | — | 8.23±1.33 | 10.31±1.40 | 7.09±1.61 | 2.26±0.80 |
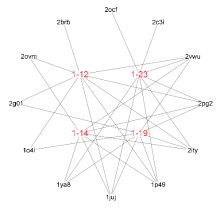
| Compd. | Pharma model (PDB ID) | Normalized fit score |
|---|---|---|
| 1-12 | 1p49 | 0.996 |
| 2vwu | 0.996 | |
| 2brb | 0.988 | |
| 2pg2 | 0.980 | |
| 1juj | 0.963 | |
| 2g01 | 0.956 | |
| 2ity | 0.935 | |
| 2ovm | 0.929 | |
| 1o4i | 0.906 | |
| 1-14 | 1p49 | 0.996 |
| 2vwu | 0.995 | |
| 2pg2 | 0.979 | |
| 1juj | 0.977 | |
| 1ya8 | 0.957 | |
| 2ovm | 0.944 | |
| 2ity | 0.925 | |
| 1-19 | 1p49 | 0.996 |
| 2vwu | 0.994 | |
| 2pg2 | 0.980 | |
| 1juj | 0.971 | |
| 1ya8 | 0.971 | |
| 2g01 | 0.946 | |
| 2ity | 0.928 | |
| 1o4i | 0.903 | |
| 1-23 | 1p49 | 0.995 |
| 2vwu | 0.993 | |
| 2c3i | 0.987 | |
| 2pg2 | 0.979 | |
| 1ya8 | 0.972 | |
| 1juj | 0.967 | |
| 2ocf | 0.942 | |
| 2ity | 0.930 |
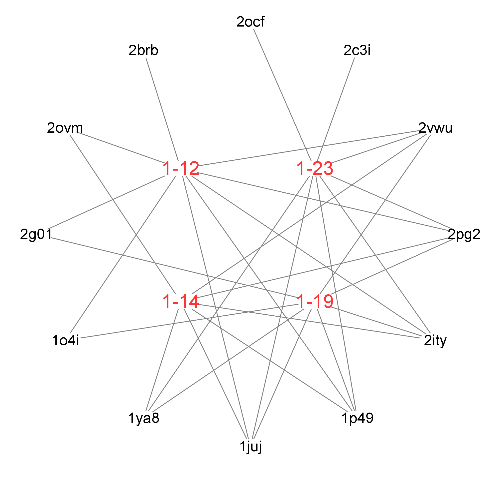
| Compd. | Pharma model (PDB ID) | Normalized fit score |
|---|---|---|
| 1-12 | 1p49 | 0.996 |
| 2vwu | 0.996 | |
| 2brb | 0.988 | |
| 2pg2 | 0.980 | |
| 1juj | 0.963 | |
| 2g01 | 0.956 | |
| 2ity | 0.935 | |
| 2ovm | 0.929 | |
| 1o4i | 0.906 | |
| 1-14 | 1p49 | 0.996 |
| 2vwu | 0.995 | |
| 2pg2 | 0.979 | |
| 1juj | 0.977 | |
| 1ya8 | 0.957 | |
| 2ovm | 0.944 | |
| 2ity | 0.925 | |
| 1-19 | 1p49 | 0.996 |
| 2vwu | 0.994 | |
| 2pg2 | 0.980 | |
| 1juj | 0.971 | |
| 1ya8 | 0.971 | |
| 2g01 | 0.946 | |
| 2ity | 0.928 | |
| 1o4i | 0.903 | |
| 1-23 | 1p49 | 0.995 |
| 2vwu | 0.993 | |
| 2c3i | 0.987 | |
| 2pg2 | 0.979 | |
| 1ya8 | 0.972 | |
| 1juj | 0.967 | |
| 2ocf | 0.942 | |
| 2ity | 0.930 |
| [1] |
Torre, L. A.; Bray, F.; Siegel, R. L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. CA-Cancer J. Clin. 2015, 65, 87.
doi: 10.3322/caac.21262 |
| [2] |
Sung, H.; Ferlay, J.; Siegel, R. L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. CA-Cancer J. Clin. 2021, 71, 209.
doi: 10.3322/caac.v71.3 |
| [3] |
Dickens, E.; Ahmed, S. Surgery (Oxford) 2018, 36, 134.
doi: 10.1016/j.mpsur.2017.12.002 |
| [4] |
Jagoe, K. N. R. T.; Abalo, R. Front. Pharmacol. 2018, 9, 245.
doi: 10.3389/fphar.2018.00245 |
| [5] |
Janganati, V.; Ponder, J.; Thakkar, S.; Thakkar, S.; Jordan, C. T.; Crooks, P. A. Bioorg. Med. Chem. 2017, 25, 3694.
|
| [6] |
Pattabiraman, V. R.; Bode, J. W. Nature 2011, 480, 471.
doi: 10.1038/nature10702 |
| [7] |
Kumari, S.; Carmona, A. V.; Tiwari, A. K.; Trippier, P. C. J. Med. Chem. 2020, 63, 12290
doi: 10.1021/acs.jmedchem.0c00530 |
| [8] |
Vlaar, C. P.; Castillo-Pichardo, L.; Medina, J. I.; Marrero-Serra, C. M.; Vélez, E; Ramos, Z.; Hernández, E. Bioorg. Med. Chem. 2018, 26, 884.
doi: 10.1016/j.bmc.2018.01.003 |
| [9] |
Rai, U. S.; Isloor, A. M.; Shetty, P.; Pai, K. S. R.; Fun, H. K. Arabian J. Chem. 2015, 8, 317.
doi: 10.1016/j.arabjc.2014.01.018 |
| [10] |
Shao, P. P.; Ok, D.; Fisher, M. H.; Garcia, M. L.; Kaczorowski, G. J.; Li, C.; Lyons, K. A.; Martin, W. J.; Meinke, P. T.; Priest, B. T.; Smith, M. M.; Wyvratt, M. J.; Ye, F.; Parsons, W. H. Bioorg. Med. Chem. Lett. 2005, 15, 1901.
doi: 10.1016/j.bmcl.2005.02.002 |
| [11] |
Tarzia, G.; Shiatti, P.; Selva, D.; Favara, D.; Ceriani, S. Eur. J. Med. Chem. 1976, 11, 263.
|
| [12] |
Hickey, S. M.; Ashton, T. D.; Khosa, S. K.; Robson, R. N.; White, J. M.; Li, J.; Nation, R. L.; Yu, H. Y.; Elliott, A. G.; Butler, M. S.; Huang, J. X.; Cooper, M. A.; Pfeffer, F. M Org. Biomol. Chem. 2015, 22, 6255.
|
| [13] |
Saudi, M.; Zmurko, J.; Kaptein, S.; Rpzenski, J.; Gadakh, B.; Chaltin, P.; Marchand, A.; Neyts, J.; Aerschot, A. V. Eur. J. Med. Chem. 2016, 12, 158.
|
| [14] |
Tohnishi, M.; Nakao, H.; Furuya, T.; Seo, A.; Kodama, H.; Tsubata, K.; Fujioka, S.; Kodama, H.; Hirooka, T.; Nishimatsu, T. J. Pestic. Sci. 2005, 30, 354.
doi: 10.1584/jpestics.30.354 |
| [15] |
Galano, A.; Alvarez-Idaboy, J. R.; Vivier-Bunge, A. Theor. Chem. Acc. 2007, 118, 597.
doi: 10.1007/s00214-007-0353-z |
| [16] |
Talele, T. T. J. Med. Chem. 2016, 59, 8712.
doi: 10.1021/acs.jmedchem.6b00472 |
| [17] |
Julius, D. A. Am. J. Psychiatry 1979, 136, 782.
doi: 10.1176/ajp.136.6.782 |
| [18] |
Yakes, F. M.; Chen, J.; Tan, J.; Yamaguchi, K.; Shi, Y.; Yu, P.; Qian, F.; Chu, F.; Bentzien, F.; Cancilla, B.; Orf, J.; You, A.; Laird, A. D.; Engst, S.; Lee, L.; Lesch, J.; Chou, Y.-C.; Joly, A. H. J. Clin. Oncol. 2017, 35, 591.
doi: 10.1200/JCO.2016.70.7398 |
| [19] |
Chopra, N.; Nathan, P. D. Expert Rev. Anticancer Ther. 2015, 15, 749.
doi: 10.1586/14737140.2015.1060127 |
| [20] |
Scheciiter, M. S.; Sullivan, W. N.; Schoof, H. F.; Maddock, D. R.; Amyx, C. M.; Porter, J. E. J. Med. Entomol. 1974, 11, 231.
pmid: 4851258 |
| [21] |
Matsui, K.; Kido, Y.; Watari, R.; Kashima, Y.; Yoshida, Y.; Shuto, S. Chem. Eur. J. 2017, 23, 3034.
doi: 10.1002/chem.201604946 |
| [22] |
Lavedan, C.; Forsberg, M.; Gentile, A. J. Neuropharmacology 2015, 91, 142.
doi: 10.1016/j.neuropharm.2014.12.004 |
| [23] |
Yamaguchi, K.; Kazuta, Y.; Hirano, K.; Yamada, S.; Matsuda, A.; Shuto, S. Bioorg. Med. Chem. 2008, 16, 8875.
doi: 10.1016/j.bmc.2008.08.061 |
| [24] |
Martin, S. F.; Dorsey, G. O.; Gane, T.; Hillier, M. C.; Kessler, H.; Baur, M.; Mathä, B.; Erickson, J. W.; Bhat, T. N.; Munshi, S.; Gulnik, S. V.; Topol, I. A. J. Med. Chem. 1998, 41, 1581.
pmid: 9572884 |
| [25] |
Chen, Y.; Le, V.; Xu, X.; Shao, X.; Liu, J.; Li, Z. Bioorg. Med. Chem. Lett. 2014, 24, 3948.
doi: 10.1016/j.bmcl.2014.06.041 |
| [26] |
Ozoe, Y.; Kita, T.; Ozoe, F.; Nakao, T.; Sato, K.; Hirase, K. Pestic. Biochem. Physiol. 2013, 107, 285.
doi: 10.1016/j.pestbp.2013.09.005 |
| [27] |
Shang, J.; Liu, Q.; Wang, B.; Li, Z. Chin. J. Org. Chem. 2019, 39, 1489. (in Chinese)
doi: 10.6023/cjoc201810025 |
|
(尚俊峰, 刘巧霞, 王宝雷, 李正名, 有机化学, 2019, 39, 1489.)
doi: 10.6023/cjoc201810025 |
|
| [28] |
Chen, K.; Liu, Q.; Ni, J.; Zhu, H.; Li, Y.; Wang, Q. Pest Manage. Sci. 2014, 71, 1503.
doi: 10.1002/ps.3954 |
| [29] |
Duan, J. J.-W.; Lu, Z.; Jiang, B.; Stachura, S.; Weigelt, C. A.; Sack, J. S.; Khan, J.; Ruzanov, M.; Galella, M. A.; Wu, D.-R.; Yarde, M.; Shen, D.-R.; Shuster, D. J.; Borowski, V.; Xie, J. H.; Zhang, L.; Vanteru, S.; Gupta, A. K.; Mathur, A.; Zhao, Q.; Foster, W.; Salter-Cid, L. M.; Carter, P. H.; Dhar, T. G. M. ACS Med. Chem. Lett. 2019, 10, 367.
doi: 10.1021/acsmedchemlett.9b00010 |
| [30] |
Li, Z.; Xu, X.; Chen, Y.; Liu, J.; Li, W. CN 103435562, 2013. (in Chinese)
|
| [31] |
Li, Z.; Chen, Y.; Liu, A.; Li, Y.; Wang, B.; Pan, L.; Wan, Y.; Liu, J.; Chen, W. CN 104402785, 2015. (in Chinese)
|
| [32] |
Lepri, S.; Goracci, L.; Valeri, A.; Cruciani, G. Eur. J. Med. Chem. 2016, 121, 658.
doi: 10.1016/j.ejmech.2016.06.006 |
| [33] |
Simpkins, L. M.; Bolton, S.; Pi, Z.; Sutton, J. C.; Kwon, C.; Zhao, G.; Magnin, D. R.; Augeri, D. J.; Gungor, T.; Rotella, D. P.; Sun, Z.; Liu, Y.; Slusarchyk, W. S.; Marcinkeviciene, J.; Robertson, J. G.; Wang, A.; Robl, J. A.; Atwal, K. S.; Zahler, R. L.; Parker, R. A.; Kirby, M. S.; Hamann, L. G. Bioorg. Med. Chem. Lett. 2007, 17, 6476.
pmid: 17937986 |
| [34] |
Wang, F.; Xu, X. Wang, F; Peng, L.; Zhang, Y.; Wang, L.; Wang, L. Lett. Org. Chem. 2015, 12, 741.
doi: 10.2174/1570178612666150907204813 |
| [35] |
Milewska, M. J.; Gdaniec, M.; Poloński, T. Tetrahedron: Asymmetry 1996, 7, 3169.
doi: 10.1016/0957-4166(96)00419-3 |
| [36] |
Beutner, G. L.; Young, I. S.; Davies, M. L.; Hickey, M. R.; Park, H.; Stevens, J. M.; Ye, Q. Org. Lett. 2018, 20, 4218.
doi: 10.1021/acs.orglett.8b01591 |
| [37] |
Liu, X.; Ouyang, S.; Yu, B.; Liu, Y.; Huang, K.; Gong, J.; Zheng, S.; Li, Z.; Li, H.; Jiang, H. Nucleic Acids Res. 2010, 38, 609.
|
| [38] |
Wang, X.; Pan, C.; Gong, J.; Liu, X.; Li, H. J. Chem. Inf. Model. 2016, 56, 1175.
doi: 10.1021/acs.jcim.5b00690 pmid: 27187084 |
| [39] |
Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. Nucleic Acids Res. 2017, 45, 356.
doi: 10.1093/nar/gkx374 pmid: 28472422 |
| [40] |
Ferrante, P.; Messali, S.; Meroni, G.; Ballabio, A. Eur. J. Hum. Genet. 2002, 10, 813.
doi: 10.1038/sj.ejhg.5200887 pmid: 12461688 |
| [41] |
Hernandez-Guzman, F. G.; Higashiyama, T.; Pangborn, W.; Osawa, Y.; Ghosh, D. J. Biol. Chem. 2003, 278, 22989.
pmid: 12657638 |
| [42] |
Geisler, J.; Sasano, H.; Chen, S.; Purohit, A. J. Steroid Biochem. Mol. Biol. 2011, 125, 39.
doi: 10.1016/j.jsbmb.2011.02.002 |
| [43] |
Purohit, A.; Foster, P. A. J. Endocrinol. 2012, 212, 99.
doi: 10.1530/JOE-11-0266 pmid: 21859802 |
| [44] |
Leese, M. P.; Hejaz, H. A. M.; Mahon, M. F.; Newman, S. P.; Purohit, A.; Reed, M. J.; Potter, B. V. L. J. Med. Chem. 2005, 48, 5243.
doi: 10.1021/jm050066a |
| [45] |
A. Purohit, Woo, L. W. L.; Potter, B. V. L. Cancer Res. 2000, 60, 3394.
pmid: 10910045 |
| [46] |
Kertesz, N.; Krasnoperov, V.; Reddy, R.; Leshanski, L.; Kumar, S. R.; Zozulya, S.; Gill, P. S. Blood 2006, 107, 2330.
doi: 10.1182/blood-2005-04-1655 |
| [47] |
Pasquale, E. B. Nat. Rev. Cancer 2010, 10, 165.
doi: 10.1038/nrc2806 pmid: 20179713 |
| [48] |
Dohle, W.; Jourdan, F. L.; Menchon, G.; Prota, A. E.; Foster, P. A.; Mannion, P.; Hamel, E.; Thomas, M. P.; Kasprzyk, P. G.; Ferrandis, E.; Steinmetz, M. O.; Leese, M. P.; Potter, B. V. L. J. Med. Chem. 2018, 61, 1031.
doi: 10.1021/acs.jmedchem.7b01474 |
| [49] |
Maltais, R.; Djiemeny, A. N.; Roy, J.; Barbeau, X.; Lambert, J.-P.; Poirier, D. Bioorg. Med. Chem. 2020, 28, 115368.
doi: 10.1016/j.bmc.2020.115368 |
| [1] | Simin Wu, Jiaxin Tang, Yujia Zhou, Xuetao Xu, Haoxing Zhang, Shaohua Wang. α-Glucosidase Inhibition Research of Derivatives Based on 2β-Acetoxyferruginol Scaffold Excluding Acetic Acid Group [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 613-621. |
| [2] | Feng Wang, Yu Chen, Hongyan Pei, Jing Zhang, Lixin Zhang. Design, Synthesis and Antifungal Activities of Novel 1,2,4-Oxadiazole Derivatives Containing Piperidine [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2826-2836. |
| [3] | Min Liu, Dongyan Yang, Yumei Xiao, Wangcang Su, Fenghai Zhao, Qin Zhaohai .. Synthesis and Bioactivities of 5-Nitroimino-[1,4-2H]-1,2,4-triazolines as Olefin-Imidacloprid Mimics [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2790-2799. |
| [4] | Zhipeng Liang, Hao Ye, Haibin Zhang, Guomin Jiang, Xinxing Wu. Ring Opening Amination of gem-Difluorocyclopropanes with Cyclobutanone Hydrazones [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1483-1491. |
| [5] | Huan Xu, Hongfei Wu, Xiaoming Zhang, Xingxing Lu, Tengda Sun, Yue Qi, Yufan Lin, Xinling Yang, Li Zhang, Yun Ling. Design, Synthesis and Bioactivity of Sulfonyl Hydrazides and Hydrazides Containing Fragment 1,2,3,4-Tetrahydroisoquinoline [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 725-733. |
| [6] | Yueling Liu, Xinxin Zhong, Ganbing Zhang. Density Functional Theory Study for Exploring the Mechanisms of the [3+2] Cycloaddition Reactions between 1-R-3-Phenylpropylidenecyclopropane (R=Me/H) and Furfural Catalyzed by Pd(0) [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 660-667. |
| [7] | Changxing Sun, Fuhao Zhang, Huan Zhang, Penghui Li, Lin Jiang. Design, Synthesis, Fungicidal Activity and Molecular Docking Study of Novel 2-(1-Methyl-1H-pyrazol-4-yl)pyrimidine-4-carboxamides [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 229-235. |
| [8] | Changkai Wang, Tengda Sun, Xuebo Zhang, Xinling Yang, Xingxing Lu, Huan Xu, Fasheng Shi, Li Zhang, Yun Ling. Design, Synthesis and Bioactivity of Novel Fluoropyrazole Hydrazides [J]. Chinese Journal of Organic Chemistry, 2022, 42(5): 1527-1536. |
| [9] | Xiu Wang, Wengui Duan, Guishan Lin, Baoyu Li, Wenjing Zhang, Fuhou Lei. Synthesis, Antifungal Activity, Three-Dimensional Quantitative Structure-Activity Relationship and Molecular Docking Study of 4-Acyl-3-amino-1,2,4-triazole-thioether Derivatives Containing Natural Pinene Structure [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 871-883. |
| [10] | Yuanfang Kong, Bin Yang, Yan Zhuang, Jingyu Zhang, Demei Sun, Chunhong Dong. Research Progress on the Synthesis and Structure-Activity Relationship of Five Hypoglycemic Active Heterocycles Based on Dipeptidyl Peptidase 4 (DPP-4) Target Design [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 770-784. |
| [11] | Yucheng Cui, Meihua Chen, Guishan Lin, Wengui Duan, Qingmin Li, Renxuan Zou, Bo Cen. Synthesis, Antifungal Activity and Molecular Docking Study of 1,3,4-Thiadiazole-Urea Compounds Containing gem-Dimethylcyclopropane Ring Structure [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3784-3797. |
| [12] | Yingjun Li, Ledi Lin, Jihong Liu, Lixin Gao, Li Sheng, Kun Jin, Xuejie Liu, Hongjing Yang, Jia Li. Synthesis and Protein Tyrosine Phosphatase 1B (PTP1B) Inhibitory Activity Evaluation of Novel N-Acylhydrazone Derivatives Containing Carbazole and Aromatic Ring/Aromatic Fused Heterocycle [J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3593-3607. |
| [13] | Yingjun Li, Ledi Lin, Kun Jin, Lixin Gao, Li Sheng, Jihong Liu, Jia Li. Synthesis and Protein Tyrosine Phosphatase 1B (PTP1B) Inhibitory Activity Evaluation of Novel Arylaminoacetylhydrazone Derivatives Containing Carbazole Moiety [J]. Chinese Journal of Organic Chemistry, 2021, 41(8): 3157-3170. |
| [14] | Shihu Qian, Yuanzheng Huang, Jiaming Li, Yanchun Zhang, Bin Zhang, Fan Jin. Synthesis and Anti-proliferative Activity of Indole-2-amide Derivatives as Cyclooxygenase-2/5-lipoxygenase (COX-2/5-LOX) Dual Inhibitors [J]. Chinese Journal of Organic Chemistry, 2021, 41(4): 1631-1638. |
| [15] | Chenhao Xu, Yunpeng Gong, Yaxin Chen, Qimeng Song, Jiao Li, Yichao Zheng, Wen Li, Kai Sun, Hongmin Liu. Design, Synthesis and Activity Evaluation of Novel Bromodomain-Containing Protein 4 (BRD4) Small Molecule Inhibitor Based on ABBV-075 [J]. Chinese Journal of Organic Chemistry, 2021, 41(4): 1712-1721. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||