Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (3): 770-784.DOI: 10.6023/cjoc202107001 Previous Articles Next Articles
REVIEWS
孔媛芳a, 杨彬a, 庄严a, 张京玉a, 孙德梅a,*( ), 董春红b,*(
), 董春红b,*( )
)
收稿日期:2021-07-01
修回日期:2021-09-30
发布日期:2021-11-09
通讯作者:
孙德梅, 董春红
基金资助:
Yuanfang Konga, Bin Yanga, Yan Zhuanga, Jingyu Zhanga, Demei Suna( ), Chunhong Dongb(
), Chunhong Dongb( )
)
Received:2021-07-01
Revised:2021-09-30
Published:2021-11-09
Contact:
Demei Sun, Chunhong Dong
Supported by:Share
Yuanfang Kong, Bin Yang, Yan Zhuang, Jingyu Zhang, Demei Sun, Chunhong Dong. Research Progress on the Synthesis and Structure-Activity Relationship of Five Hypoglycemic Active Heterocycles Based on Dipeptidyl Peptidase 4 (DPP-4) Target Design[J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 770-784.
| Compd. | IC50/(mg•L–1) | |
|---|---|---|
| α-Glucosidase inhibitory activity | AGEs forming free radical activity | |
| 1a | >1000 | 5.247±0.519 |
| 1b | >1000 | 6.567±0.456 |
| 1c | >1000 | 3.239±0.713 |
| 1d | >1000 | 2.346±0.188 |
| 2a | >1000 | 4.223±0.268 |
| 2b | 0.469±0.042 | 2.453±0.069 |
| 2c | 0.250±0.042 | 1.132±0.141 |
| 2d | 1.020±0.062 | 0.623±0.016 |
| Acarbose | 0.042±0.002 | — |
| Aminoguanidine Hydrochloride | — | 28.255±0.657 |
| Compd. | IC50/(mg•L–1) | |
|---|---|---|
| α-Glucosidase inhibitory activity | AGEs forming free radical activity | |
| 1a | >1000 | 5.247±0.519 |
| 1b | >1000 | 6.567±0.456 |
| 1c | >1000 | 3.239±0.713 |
| 1d | >1000 | 2.346±0.188 |
| 2a | >1000 | 4.223±0.268 |
| 2b | 0.469±0.042 | 2.453±0.069 |
| 2c | 0.250±0.042 | 1.132±0.141 |
| 2d | 1.020±0.062 | 0.623±0.016 |
| Acarbose | 0.042±0.002 | — |
| Aminoguanidine Hydrochloride | — | 28.255±0.657 |
| Compd. | R1 | R2 | n | Decrease in blood glucose/% | |
|---|---|---|---|---|---|
| 18 h | 21 h | ||||
| 4a | Me | H | 0 | -7.0±4.8 | 3.6±0.7 |
| 4b | Me | H | 1 | 24.7±9.1 | 27.6±5.9 |
| 4c | Me | H | 2 | -5.6±6.2 | 13.0±9.3 |
| 4d | Me | H | 3 | -3.0±6.1 | -9.3±18.3 |
| 4e | H | H | 1 | 13.9±12.7 | 7.4±3.0 |
| 4f | Et | H | 1 | -19.5±8.7 | 2.4±8.3 |
| 4g | Ph | H | 1 | 3.9±5.6 | -3.4±22.4 |
| 4h | 4-ClC6H4CH2 | H | 1 | 24.5±8.2 | -8.5±19.0 |
| 4i | 4-PhC6H4CH2 | H | 1 | 3.8±13.4 | 11.4±6.0 |
| 4j | Me | Cl | 1 | 37.1±7.7 | 47.6±6.9 |
| Compd. | R1 | R2 | n | Decrease in blood glucose/% | |
|---|---|---|---|---|---|
| 18 h | 21 h | ||||
| 4a | Me | H | 0 | -7.0±4.8 | 3.6±0.7 |
| 4b | Me | H | 1 | 24.7±9.1 | 27.6±5.9 |
| 4c | Me | H | 2 | -5.6±6.2 | 13.0±9.3 |
| 4d | Me | H | 3 | -3.0±6.1 | -9.3±18.3 |
| 4e | H | H | 1 | 13.9±12.7 | 7.4±3.0 |
| 4f | Et | H | 1 | -19.5±8.7 | 2.4±8.3 |
| 4g | Ph | H | 1 | 3.9±5.6 | -3.4±22.4 |
| 4h | 4-ClC6H4CH2 | H | 1 | 24.5±8.2 | -8.5±19.0 |
| 4i | 4-PhC6H4CH2 | H | 1 | 3.8±13.4 | 11.4±6.0 |
| 4j | Me | Cl | 1 | 37.1±7.7 | 47.6±6.9 |
| Entry | FPG/(mol•L–1) | 2 h PBG/(mol•L–1) | |||
|---|---|---|---|---|---|
| Before treatment | Post treatment | | Before treatment | Post treatment | |
| 1 | 7.43±0.70 | 5.30±0.39 | 11.33±1.24 | 6.80±0.53 | |
| t | 0.569 | 12.010 | 0.105 | 8.000 | |
| P | 0.285 | 0.000 | 0.458 | 0.000 | |
| Entry | FPG/(mol•L–1) | 2 h PBG/(mol•L–1) | |||
|---|---|---|---|---|---|
| Before treatment | Post treatment | | Before treatment | Post treatment | |
| 1 | 7.43±0.70 | 5.30±0.39 | 11.33±1.24 | 6.80±0.53 | |
| t | 0.569 | 12.010 | 0.105 | 8.000 | |
| P | 0.285 | 0.000 | 0.458 | 0.000 | |
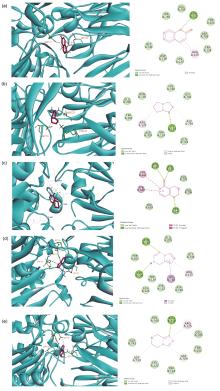
| Entry | Compound | Binding energy/(kJ•mol–1) |
|---|---|---|
| 1 | a | –25.62 |
| 2 | g | –22.68 |
| 3 | j | –22.68 |
| 4 | b | –21.84 |
| 5 | i | –21.42 |
| 6 | e | –21 |
| 7 | l | –20.58 |
| 8 | h | –20.16 |
| 9 | d | –19.32 |
| 10 | c | –17.64 |
| 11 | k | –17.64 |
| 12 | f | –13.86 |
| Entry | Compound | Binding energy/(kJ•mol–1) |
|---|---|---|
| 1 | a | –25.62 |
| 2 | g | –22.68 |
| 3 | j | –22.68 |
| 4 | b | –21.84 |
| 5 | i | –21.42 |
| 6 | e | –21 |
| 7 | l | –20.58 |
| 8 | h | –20.16 |
| 9 | d | –19.32 |
| 10 | c | –17.64 |
| 11 | k | –17.64 |
| 12 | f | –13.86 |

| [1] |
Chakraborty, R.; Ahmed, A. B.; Saha, D. Int. J. Curr. Pharm. Res. 2019, 4, 60.
|
| [2] |
Wang, L. M.; Gao, P.; Zhang, M.; Huang, Z. J.; Zhang, D. D.; Deng, Q.; Li, Y. C.; Zhao, Z. P.; Qin, X. Y.; Jin, D. Y.; Zhou, M. G.; Tang, X.; Hu, Y. H.; Wang, L. H. J. Am. Med. Assoc. 2017, 317, 2515.
doi: 10.1001/jama.2017.7596 |
| [3] |
Zimmet, P.; Shaw, J.; Alberti, K. G. M. M. Diabetic Med. 2003, 20, 693.
pmid: 12925046 |
| [4] |
Shen, W. Chin. J. Misdiagn 2011, 11, 8845. (in Chinese)
|
|
(谌卫, 中国误诊学杂志, 2011, 11, 8845.)
|
|
| [5] |
(a) Liakos, C. I.; Papadopoulos, D. P.; Sanidas, E. A.; Markou, M. I.; Hatziagelaki, E. E.; Grassos, C. A.; Velliou, M. L.; Barbetseas, J. D. Am. J. Cardiovasc. Drugs 2020, 21, 262.
|
|
(b) Sun, Z. G.; Li, Z. N.; Zhu, H. L. Mini-Rev. Med. Chem. 2020, 20, 1709.
doi: 10.2174/1389557520666200628032507 |
|
| [6] |
Xiong, L. J.; Jin, Y.; Fang, Y. Y. J. Shenyang Pharm. Univ. 2020, 37, 181. (in Chinese)
|
|
(熊丽娟, 金一, 房元英, 沈阳药科大学学报, 2020, 37, 181.)
|
|
| [7] |
Deacon, C. F. Diabetes 2004, 53, 2181.
doi: 10.2337/diabetes.53.9.2181 |
| [8] |
Tang, Y. Z.; Tian, L. L.; Ni, C. L. Med. Recapitulate 2018, 24, 770. (in Chinese)
|
|
(汤云昭, 田琳琳, 倪长霖, 医学综述, 2018, 24, 770.)
|
|
| [9] |
Dong, P. J. Qiqihar Med. Univ. 2019, 40, 218.
|
| [10] |
Havale, S. H.; Pal, M. Bioorg. Med. Chem. 2009, 17, 1783.
doi: 10.1016/j.bmc.2009.01.061 |
| [11] |
Rosenstock, J.; Perkovic, V.; Johansen, O. E.; Mark, E. Cooper, M. E.; Kahn, S. E.; Marx, N.; Alexander, J. H.; Pencina, M.; Toto, R. D.; Wanner, C.; Zinman, B.; Woerle, H. J.; Baanstra, D.; Pfarr, E.; Schnaidt, S.; Meinicke, T.; George, J. T.; Eynatten, M. V.; McGuire, D. K. J. Am. Med. Assoc. 2018. 321, 69.
doi: 10.1001/jama.2018.18269 |
| [12] |
Hu, Y. H.; Sun, J.; Yang, J.; Wang, X. J. Chem. Res. 2018, 29, 357. (in Chinese)
|
|
(胡玉恒, 孙捷, 杨洁, 王晓静, 化学研究, 2018, 29, 357.)
|
|
| [13] |
Thomas, D. A.; Bork, B.; Philip, A. B.; Leonard, J. B.; Cheon, S. H.; Rhonda, O. D.; Jay, B. F.; William, S. F.; James, D. F.; Jiaping, G.; Douglas, C. K.; Gerald, G. K.; Christina, L. L.; Jeffrey, N.; Ronald, S.; Howard, C. S. J. Med. Chem. 1998, 41, 4.
|
| [14] |
Aicher, T. D; Balkan, B.; Bell, P. A.; Brand, L. J.; Cheon, S. H.; Deems, R. O.; Fell, J. B.; Fillers, W. S.; Fraser, J. D; Gao, J.; Knorr, D. C.; Kahle, G. G.; Leone, C. L.; Nadelson, J.; Simpson, R.; Smith, H. C. J. Med. Chem. 1998, 41, 4556.
pmid: 9804695 |
| [15] |
Blank, B.; DiTullio, N. W.; Krog, A. J.; Saunders, H. L. J. Med. Chem. 1978, 21, 489.
pmid: 660598 |
| [16] |
Meltzer-Mats, E.; Babai-Shani, G.; Pasternak, L.; Uritsky, N.; Getter, T.; Viskind, O.; Eckel, J.; Cerasi, E.; Senderowitz, H.; Sasson, S.; Gruzman, A. J. Med. Chem. 2013, 56, 5335.
doi: 10.1021/jm4001488 pmid: 23750537 |
| [17] |
Oguchi, M.; Wada, K.; Honma, H.; Tanaka, A.; Kaneko, T.; Sakakibara, S.; Ohsumi, J.; Serizawa, N.; Fujiwara, T.; Horikoshi, H.; Fujita, T. J. Med. Chem. 2000, 43, 3052.
pmid: 10956213 |
| [18] |
Qiao, Y. B.; Xu, Q. Q.; Feng, W. Y.; Tao, L.; Li, X. N.; Liu, J. J.; Zhu, H. C.; Lu, Y. Y.; Wang, J. P.; Qi, C. X.; Xue, Y. B.; Zhang, Y. H. J. Nat. Prod. 2019, 82, 2925.
doi: 10.1021/acs.jnatprod.9b00188 |
| [19] |
Blank, B.; DiTullio, N. W.; Deviney, L.; Roberts, J. T.; Magnani, A.; Billig, M.; Saunders, H. L. J. Med. Chem. 1977, 20, 1572.
pmid: 592321 |
| [20] |
Blank, B.; DiTullio, N. W.; Krog, A. J.; Saunders, H. L. J. Med. Chem. 1979, 22, 840.
pmid: 448683 |
| [21] |
Li, C. J.; Le, Z. P.; Zhao, C. S. Chin. J. New Drugs 2014, 23, 2195. (in Chinese)
|
|
(李翠娟, 乐治平, 赵传生, 中国新药杂志, 2014, 23, 2195.)
|
|
| [22] |
Li, Z. Y.; Wang, J. T.; Gu, Y. Q.; Tang, L. Chem. Reagents 2013, 35, 114. (in Chinese)
|
|
(李志燕, 王建塔, 古元琴, 汤磊, 化学试剂, 2013, 35, 114.)
|
|
| [23] |
Zhang, C. L. Heilongjiang Med. J. 2019, 32, 1095. (in Chinese)
|
|
(张春玲, 黑龙江医药, 2019, 32, 1095.)
|
|
| [24] |
Veselinovic, J. B.; Veselinovic, A. M.; Vitnik, Z. J.; Vitnik, V. D.; Nikolic, G. M. Chem. Biol. 2014, 214, 49.
|
| [25] |
Arora, R. K.; Kaur, N.; Bansal, Y.; Bansal, G. Acta Pharm. Sin. B 2014, 4, 368.
doi: 10.1016/j.apsb.2014.07.001 |
| [26] |
Mahajan, D. H.; Pannecouque, C.; De, C. E.; Chikhalia, K. H. Arch. Pharm. (Weinheim, Ger.) 2009, 342, 281.
doi: 10.1002/ardp.v342:5 |
| [27] |
Sabry, N. M.; Mohamed, H. M.; Khattab, E. S.; Motlaq, S. S.; El- Agrody, A. M. Eur. J. Med. Chem. 2011, 46, 765.
doi: 10.1016/j.ejmech.2010.12.015 |
| [28] |
Miri, R.; Nejati, M.; Saso, L.; Khakdan, F.; Parshad, B.; Mathur, D.; Parmar, V. S.; Bracke, M. E.; Prasad, A. K.; Sharma, S. K.; Firuzi, O. Pharm. Biol. 2016, 54, 105.
doi: 10.3109/13880209.2015.1016183 |
| [29] |
Manvar, A.; Malde, A.; Verma, J.; Virsodia, V.; Mishra, A.; Upadhyay, K.; Acharya, H.; Coutinho, E.; Shah, A. Eur. J. Med. Chem. 2008, 43, 2395.
doi: 10.1016/j.ejmech.2008.01.016 pmid: 18328603 |
| [30] |
Yang, L. S.; Wang, Y.; Wang, E. H.; Yang, J.; Pan, X.; Liao, X.; Yang, X. S. Synth. Commun. 2020, 50, 3080.
doi: 10.1080/00397911.2020.1792498 |
| [31] |
Shakil, M.; Meguerdichian, A. G.; Tasnim, H.; Shirazi, A. A.; Seraji, M. S.; Suib, S. L. Inorg. Chem. 2019, 58, 5703.
doi: 10.1021/acs.inorgchem.9b00053 pmid: 30964675 |
| [32] |
Song, D.; Wang, C. M.; Ye, Z. P.; Xia, P. J.; Deng, Z. X.; Xiao, J. A.; Xiang, H. Y.; Yang, H. J. Org. Chem. 2019, 84, 7480.
doi: 10.1021/acs.joc.9b00715 pmid: 31062593 |
| [33] |
Vashishtha, M.; Mishra, M.; Shah, D. O. Green Chem. 2016, 18, 1339.
doi: 10.1039/C5GC01966D |
| [34] |
Konrádová, D.; Kozubíková, H.; Doležal, K.; Pospíšil, J. Eur. J. Org. Chem. 2017, 2017, 5204.
doi: 10.1002/ejoc.201701021 |
| [35] |
Guerrero-Analco, J.; Medina-Campos, O.; Brindis, F.; Bye, R.; Pedraza-Chaverri, J.; Navarrete, A.; Mata, R. Phytochemistry 2007, 68, 2087.
pmid: 17575991 |
| [36] |
Soares, J.; Espadinha, M.; Raimundo, L.; Ramos, H.; Gomes, A. S.; Gomes, S.; Loureiro, J. B.; Inga, A.; Reis, F.; Gomes, C.; Santos, M. M. M.; Saraiva, L. Mol. Oncol. 2017, 11, 612.
doi: 10.1002/1878-0261.12051 pmid: 28296148 |
| [37] |
Bao, B.; Bai, S.; Fan, J.; Su, J.; Wang, W.; Yu, D. Dyes Pigm. 2019, 171, 107778.
doi: 10.1016/j.dyepig.2019.107778 |
| [38] |
Chen, W. L.; Wang, L. Y.; Li, Y. J. Eur. J. Org. Chem. 2020, 1, 103.
|
| [39] |
Vasylyev, M.; Alper, H. Angew. Chem., Int. Ed. 2009, 48, 1287.
doi: 10.1002/anie.200802550 |
| [40] |
Elmore, S. W.; Coghlan, M. J.; Anderson, D. D.; Pratt, J. K.; Green, B. E.; Wang, A. X.; Stashko, M. A.; Lin, C. W.; Tyree, C. M.; Miner, J. N.; Jacobson, P. B.; Wilcox, D. M.; Lane, B. C. J. Med. Chem. 2001, 44, 4481.
pmid: 11728194 |
| [41] |
Wu, C. S.; Lin, Z. M.; Wang, L. N.; Guo, D. X.; Wang, S. Q.; Liu, Y. Q.; Yuan, H. Q.; Lou, H. X. Bioorg. Med. Chem. Lett. 2011, 21, 3261.
doi: 10.1016/j.bmcl.2011.04.025 |
| [42] |
Zan, L. F.; Qin, J. C.; Zhang, Y. M.; Yao, Y. H.; Bao, H. Y.; Li, X. Chem. Pharm. Bull. (Tokyo) 2011, 59, 770.
doi: 10.1248/cpb.59.770 |
| [43] |
Gurubrahamam, R.; Gao, B. F.; Chen, Y. M.; Chan, Y. T.; Tsai, M. K.; Chen, K. Org. Lett. 2016, 18, 3098.
doi: 10.1021/acs.orglett.6b01265 pmid: 27324401 |
| [44] |
Shrestha, R.; Khanal, H. D.; Lee, Y. R. RSC Adv. 2019, 9, 17347.
doi: 10.1039/c9ra03146d |
| [45] |
Bollikolla, H. B.; Choppakatla, S.; Polam, N.; Thripuram, V. D.; Chidipudi, S. R. Asian J. Org. Chem. 2017, 6, 1598.
doi: 10.1002/ajoc.v6.11 |
| [46] |
Neve, J. E.; Wijesekera, H. P.; Duffy, S.; Jenkins, I. D.; Ripper, J. A.; Teague, S. J.; Campitelli, M.; Garavelas, A.; Nikolakopoulos, G.; Le, P. V.; Leone, P.; Pham, N. B.; Shelton, P.; Fraser, N.; Carroll, A. R.; Avery, V. M.; McCrae, C.; Williams, N.; Quinn, R. J. J. Med. Chem. 2014, 57, 1252.
doi: 10.1021/jm401321v |
| [47] |
Yetra, S. R.; Roy, T.; Bhunia, A.; Porwal, D.; Biju, A. T. J. Org. Chem. 2014, 79, 4245.
doi: 10.1021/jo500693h |
| [48] |
Zhang, C.; Jin, L.; Mondie, B.; Mitchell, S. S.; Castelhano, A. L.; Cai, W. Z.; Bergenhem, N. Bioorg. Med. Chem. Lett. 2003, 13, 1433.
|
| [49] |
Zhang, Z.; Wallace, M. B.; Feng, J.; Stafford, J. A.; Skene, R. J.; Shi, L.; Lee, B.; Aertgeerts, K.; Jennings, A.; Xu, R.; Kassel, D. B.; Kaldor, S. W.; Navre, M.; Webb, D. R.; Gwaltney, S. L. J. Med. Chem. 2011, 54, 510.
doi: 10.1021/jm101016w |
| [50] |
Cai, Z. W.; Wei, D.; Borzilleri, R. M.; Qian, L.; Kamath, A.; Mortillo, S.; Wautlet, B.; Henley, B. J.; Jeyaseelan, R. S.; Tokarski, J.; Hunt, J. T.; Bhide, R. S.; Fargnoli, J.; Lombardo, L. J. Bioorg. Med. Chem. Lett. 2008, 18, 1354.
doi: 10.1016/j.bmcl.2008.01.012 |
| [51] |
Awale, M.; Mohan, C. G. J. Mol. Graphics Modell. 2008, 26, 1169.
doi: 10.1016/j.jmgm.2007.10.008 |
| [52] |
Ke, Z.; Lu, T.; Liu, H.; Yuan, H.; Ran, T.; Zhang, Y.; Yao, S.; Xiong, X.; Xu, J.; Xu, A.; Chen, Y. J. Mol. Struct. 2014, 1067, 127.
doi: 10.1016/j.molstruc.2014.03.036 |
| [53] |
Dugar, S.; Hollinger, F. P; Kuila, B.; Arora, R.; Sen, S.; Mahajan, D. Bioorg. Med. Chem. Lett. 2015, 25, 3142.
doi: 10.1016/j.bmcl.2015.06.007 |
| [54] |
Collin, M. P.; Lobell, M.; Hubsch, W.; Brohm, D.; Schirok, H.; Jautelat, R.; Lustig, K.; Bomer, U.; Vohringer, V.; Heroult, M.; Grunewald, S.; Hess, S. H. Med. Chem. 2018, 13, 437.
|
| [55] |
Qin, L. Y.; Ruan, Z.; Cherney, R. J.; Dhar, T. G. M.; Neels, J.; Weigelt, C. A.; Sack, J. S.; Srivastava, A. S.; Cornelius, L. A. M.; Tino, J. A.; Stefanski, K.; Gu, X.; Xie, J.; Susulic, V.; Yang, X.; Yarde, C. M.; Skala, S.; Bosnius, R.; Goldstein, C.; Davies, P.; Ruepp, S.; Salter, C. L.; Bhide, R. S.; Poss, M A. Bioorg. Med. Chem. Lett. 2017, 27, 855.
doi: 10.1016/j.bmcl.2017.01.016 |
| [56] |
Shi, W.; Qiang, H.; Huang, D. D.; Bi, X. Z.; Huang, W. L.; Qian, H. Eur. J. Med. Chem. 2018, 158, 814.
doi: 10.1016/j.ejmech.2018.09.050 |
| [57] |
Holmes, J. L.; Almeida, L.; Barlaam, B.; Croft, R. A.; Dishington, A. P.; Gingipalli, L.; Hassall, L. A.; Hawkins, J. L.; Ioannidis, S.; Johannes, J. W.; McGuire, T. M.; Moore, J. E.; Patel, A.; Pike, K. G.; Pontz, T.; Wu, X. Y.; Wang, T.; Zhang, H. J.; Zheng, X. L. Synthesis 2016, 48, 1226.
doi: 10.1055/s-00000084 |
| [58] |
Jia, H.; Dai, G.; Su, W.; Xiao, K.; Weng, J.; Zhang, Z.; Wang, Q.; Yuan, T.; Shi, F.; Zhang, Z.; Chen, W.; Sai, Y.; Wang, J.; Li, X.; Cai, Y.; Yu, J.; Ren, P.; Venable, J.; Rao, T.; Edwards, J. P.; Bembenek, S. D. J. Med. Chem. 2019, 62, 4936.
doi: 10.1021/acs.jmedchem.8b02014 |
| [59] |
Falsini, M.; Squarcialupi, L.; Catarzi, D.; Varano, F.; Betti, M.; Dal, B. D.; Marucci, G.; Buccioni, M.; Volpini, R.; De, V. T.; Cavalli, A.; Colotta, V. J. Med. Chem. 2017, 60, 5772.
doi: 10.1021/acs.jmedchem.7b00457 pmid: 28590753 |
| [60] |
Jethava, D. J.; Acharya, P. T.; Vasava, M. S.; Bhoi, M. N.; Bhavsar, Z. A.; Rathwa, S. K.; Rajani, D. P.; Patel, H. D. J. Mol. Struct. 2019, 1184, 168.
doi: 10.1016/j.molstruc.2019.01.091 |
| [61] |
Guan, L. P.; Zhang, R. P.; Chang, Y.; Gan, X. X. Asian J. Chem. 2013, 25, 3660.
doi: 10.14233/ajchem |
| [62] |
Hou, Y. L.; Zhu, L. Y.; Li, Z. W.; Shen, Q.; Xu, Q. L.; Li, W.; Liu, Y. J.; Gong, P. Eur. J. Med. Chem. 2019, 163, 690.
doi: 10.1016/j.ejmech.2018.12.009 |
| [63] |
Mannam, M. R.; Devineni, S. R.; Pavuluri, C. M.; Chamarthi, N. R.; Kottapalli, R. S. P. Phosphorus, Sulfur Silicon Relat. Elem. 2019, 194, 922.
doi: 10.1080/10426507.2019.1577845 |
| [64] |
Sumran, G.; Aggarwal, R.; Mittal, A.; Aggarwal, A.; Gupta, A. Bioorg. Chem. 2019, 88, 102932.
doi: 10.1016/j.bioorg.2019.102932 |
| [65] |
Unciti-Broceta, A.; Pineda-de-las-Infantas, M. J.; Díaz-Mochón, J. J.; Romagnoli, R.; Baraldi, P. G.; Gallo, M. A.; Espinosa, A. J. Org. Chem. 2005, 70, 2878.
pmid: 15787593 |
| [66] |
Ayothiraman, R.; Bandaru, D.; Paranthaman, R.; Fenster, M.; Eastgate, M. D.; Vaidyanathan, R. Org. Process Res. Dev. 2019, 23, 2510.
doi: 10.1021/acs.oprd.9b00396 |
| [67] |
Li, C. M.S. Thesis Fudan University, Shanghai, 2010. (in Chinese)
|
|
(李超, 硕士论文, 复旦大学, 上海, 2010.)
|
|
| [68] |
Deacon, C. F.; Hughes, T. E.; Holst, J. J. Diabetes 1998, 47, 764.
pmid: 9588448 |
| [1] | Simin Wu, Jiaxin Tang, Yujia Zhou, Xuetao Xu, Haoxing Zhang, Shaohua Wang. α-Glucosidase Inhibition Research of Derivatives Based on 2β-Acetoxyferruginol Scaffold Excluding Acetic Acid Group [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 613-621. |
| [2] | Haibo Huo, Guixia Li, Shijun Wang, Chun Han, Baojun Shi, Jian Li. Novel γ-Carboline Derivatives as Antibacterial Agents: Synthesis and Antibacterial Evaluation [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 204-215. |
| [3] | Feng Wang, Yu Chen, Hongyan Pei, Jing Zhang, Lixin Zhang. Design, Synthesis and Antifungal Activities of Novel 1,2,4-Oxadiazole Derivatives Containing Piperidine [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2826-2836. |
| [4] | Min Liu, Dongyan Yang, Yumei Xiao, Wangcang Su, Fenghai Zhao, Qin Zhaohai .. Synthesis and Bioactivities of 5-Nitroimino-[1,4-2H]-1,2,4-triazolines as Olefin-Imidacloprid Mimics [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2790-2799. |
| [5] | Xin Zuo, Shinuo Xu, Zhongyang Chen, Jianfeng Yan, Yaofeng Yuan. Research Progress of Electron Transport Properties in Ferrocene- Containing Single-Molecule Junctions [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2313-2322. |
| [6] | Jinyan He, Fuyun Tian, Qingqing Wu, Yueming Zheng, Yuting Chen, Haiyan Xu, Zhengsheng Jin, Li Zhan, Xinqiang Cheng, Yueling Gu, Zhaobing Gao, Guilong Zhao. Design, Synthesis and Bioactivity of [3.3.3]Propellane-Based Voltage-Gated Calcium Channel α2δ Subunit Ligands [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2226-2238. |
| [7] | Xingzhou Liu, Mingjia Yu, Jianhua Liang. Research Progress on the Synthesis of Protoberberine Skeleton and Its Anti-inflammatory Activity [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1325-1340. |
| [8] | Huan Xu, Hongfei Wu, Xiaoming Zhang, Xingxing Lu, Tengda Sun, Yue Qi, Yufan Lin, Xinling Yang, Li Zhang, Yun Ling. Design, Synthesis and Bioactivity of Sulfonyl Hydrazides and Hydrazides Containing Fragment 1,2,3,4-Tetrahydroisoquinoline [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 725-733. |
| [9] | Changxing Sun, Fuhao Zhang, Huan Zhang, Penghui Li, Lin Jiang. Design, Synthesis, Fungicidal Activity and Molecular Docking Study of Novel 2-(1-Methyl-1H-pyrazol-4-yl)pyrimidine-4-carboxamides [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 229-235. |
| [10] | Rong Zhang, Xiang Gao, Lingling Chen, Fajun Nan. Discovery and Structure-Activity Relationship Studies of Thiazole- Oxazole Tandem Heterocyclic RNA Splicing Inhibitors [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2925-2939. |
| [11] | Changkai Wang, Tengda Sun, Xuebo Zhang, Xinling Yang, Xingxing Lu, Huan Xu, Fasheng Shi, Li Zhang, Yun Ling. Design, Synthesis and Bioactivity of Novel Fluoropyrazole Hydrazides [J]. Chinese Journal of Organic Chemistry, 2022, 42(5): 1527-1536. |
| [12] | Ming Cai, Liang Shao, Fan Yang, Jihong Zhang, Fei Yu. Design, Synthesis of Pentacyclic Triterpenoid Glucose Conjugate and in vitro Activity against Influenza Virus [J]. Chinese Journal of Organic Chemistry, 2022, 42(5): 1453-1462. |
| [13] | Siyu Zhu, Xinyu Huo, Qin Ma, Wei Chen, Jie Zhang, Liang Guo. Design, Synthesis, and Antitumor Activity of β-Carboline-Benzimidazole Hybrids [J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 1129-1135. |
| [14] | Xiu Wang, Wengui Duan, Guishan Lin, Baoyu Li, Wenjing Zhang, Fuhou Lei. Synthesis, Antifungal Activity, Three-Dimensional Quantitative Structure-Activity Relationship and Molecular Docking Study of 4-Acyl-3-amino-1,2,4-triazole-thioether Derivatives Containing Natural Pinene Structure [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 871-883. |
| [15] | Yan Zeng, Lifei Nie, Chao Niu, Aytilla Mamatjan, Khurshed Bozorov, Jiangyu Zhao, Haji Akber Aisa. Synthesis and Biological Activities of Dihydrooxazolo[5,4-d]-pyrrolo[1,2-a]pyrimidinones [J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 543-556. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||