Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (4): 1129-1135.DOI: 10.6023/cjoc202110025 Previous Articles Next Articles
ARTICLES
朱思玉a, 霍新玉a, 马芹b, 陈伟b, 张洁a,*( ), 郭亮a,*(
), 郭亮a,*( )
)
收稿日期:2021-10-18
修回日期:2021-12-23
发布日期:2022-01-11
通讯作者:
张洁, 郭亮
基金资助:
Siyu Zhua, Xinyu Huoa, Qin Mab, Wei Chenb, Jie Zhanga( ), Liang Guoa(
), Liang Guoa( )
)
Received:2021-10-18
Revised:2021-12-23
Published:2022-01-11
Contact:
Jie Zhang, Liang Guo
Supported by:Share
Siyu Zhu, Xinyu Huo, Qin Ma, Wei Chen, Jie Zhang, Liang Guo. Design, Synthesis, and Antitumor Activity of β-Carboline-Benzimidazole Hybrids[J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 1129-1135.
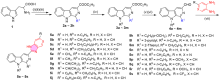

| Compd. | R1 | R9 | R | X | IC50/(μmol•L–1)±SDa | ||||
|---|---|---|---|---|---|---|---|---|---|
| A549 | BGC-823 | CT-26 | Bel-7402 | MCF-7 | |||||
| 5a | H | n-Butyl | H | CH | 32.1±2.8 | 16.1±1.4 | 17.2±1.3 | 13.3±1.1 | 12.7±0.9 |
| 5b | H | Benzyl | H | CH | 9.7±0.7 | 17.5±1.6 | 13.8±1.1 | 15.6±1.3 | 16.1±1.2 |
| 5c | H | 4-Fluorobenzyl | H | CH | 8.6±0.6 | 7.6±0.7 | 16.4±1.3 | 7.4±0.7 | 13.2±1.1 |
| 5d | H | 3-Phenylpropyl | H | CH | 15.3±1.2 | 7.4±0.5 | 11.6±0.7 | 14.6±1.1 | 12.3±0.9 |
| 5e | CH3 | n-Butyl | H | CH | 17.4±1.5 | 9.9±0.8 | 15.8±1.3 | 15.5±1.2 | 17.3±1.4 |
| 5f | CH3 | Benzyl | H | CH | 10.7±0.9 | 17.4±1.5 | 13.1±1.2 | 18.9±1.7 | 14.3±1.2 |
| 5g | CH(CH3)2 | n-Butyl | H | CH | 24.7±2.1 | 14.8±1.2 | 17.2±1.4 | 17.8±1.2 | 16.9±1.5 |
| 5h | CH(CH3)2 | Benzyl | H | CH | 19.2±1.8 | 12.9±1.2 | 15.6±1.4 | 16.3±1.5 | 18.5±1.6 |
| 5i | C6H5 | Benzyl | H | CH | 9.4±0.9 | 12.8±1.2 | 10.2±0.8 | 22.6±1.5 | 13.8±1.1 |
| 5j | C6H4(o-Cl) | n-Butyl | H | CH | 9.5±0.8 | 19.2±1.4 | 17.4±1.2 | 13.4±1.1 | 8.9±0.7 |
| 5k | C6H4(p-OCH3) | Benzyl | H | CH | 13.7±0.8 | 8.9±0.6 | 16.5±1.3 | 10.6±0.9 | 12.4±0.9 |
| 5l | 3-Pyridyl | n-Butyl | H | CH | 15.3±1.2 | 10.1±0.8 | 26.2±1.7 | 15.3±1.2 | 14.9±1.1 |
| 5m | 2-Thienyl | Benzyl | H | CH | 9.8±0.7 | 9.5±0.9 | 12.2±1.1 | 11.5±0.8 | 16.9±1.2 |
| 5n | H | n-Butyl | H | N | 14.7±1.2 | 7.5±0.5 | 18.6±1.1 | 17.8±1.4 | 23.9±1.7 |
| 5o | H | Benzyl | H | N | 21.2±1.9 | 24.7±2.1 | 32.3±2.5 | 21.1±1.8 | 12.4±1.1 |
| 5p | H | n-Butyl | OCH3 | CH | 14.6±1.3 | 24.3±1.9 | 17.3±1.6 | 27.1±1.9 | 25.7±1.6 |
| 5q | H | n-Butyl | CF3 | CH | 8.7±0.7 | 14.8±1.2 | 12.3±0.9 | 9.4±0.7 | 8.6±0.5 |
| 5r | H | Benzyl | OCH3 | CH | 14.5±1.2 | 6.2±0.4 | 19.1±0.7 | 21.4±1.8 | 33.1±2.3 |
| 5s | H | Benzyl | CF3 | CH | 10.1±0.6 | 14.3±0.8 | 18.3±0.5 | 10.1±0.7 | 4.9±0.3 |
| Cisplatin | 15.8±2.4 | 8.4±0.7 | 4.2±0.7 | 15.4±1.9 | 10.5±2.3 | ||||
| Compd. | R1 | R9 | R | X | IC50/(μmol•L–1)±SDa | ||||
|---|---|---|---|---|---|---|---|---|---|
| A549 | BGC-823 | CT-26 | Bel-7402 | MCF-7 | |||||
| 5a | H | n-Butyl | H | CH | 32.1±2.8 | 16.1±1.4 | 17.2±1.3 | 13.3±1.1 | 12.7±0.9 |
| 5b | H | Benzyl | H | CH | 9.7±0.7 | 17.5±1.6 | 13.8±1.1 | 15.6±1.3 | 16.1±1.2 |
| 5c | H | 4-Fluorobenzyl | H | CH | 8.6±0.6 | 7.6±0.7 | 16.4±1.3 | 7.4±0.7 | 13.2±1.1 |
| 5d | H | 3-Phenylpropyl | H | CH | 15.3±1.2 | 7.4±0.5 | 11.6±0.7 | 14.6±1.1 | 12.3±0.9 |
| 5e | CH3 | n-Butyl | H | CH | 17.4±1.5 | 9.9±0.8 | 15.8±1.3 | 15.5±1.2 | 17.3±1.4 |
| 5f | CH3 | Benzyl | H | CH | 10.7±0.9 | 17.4±1.5 | 13.1±1.2 | 18.9±1.7 | 14.3±1.2 |
| 5g | CH(CH3)2 | n-Butyl | H | CH | 24.7±2.1 | 14.8±1.2 | 17.2±1.4 | 17.8±1.2 | 16.9±1.5 |
| 5h | CH(CH3)2 | Benzyl | H | CH | 19.2±1.8 | 12.9±1.2 | 15.6±1.4 | 16.3±1.5 | 18.5±1.6 |
| 5i | C6H5 | Benzyl | H | CH | 9.4±0.9 | 12.8±1.2 | 10.2±0.8 | 22.6±1.5 | 13.8±1.1 |
| 5j | C6H4(o-Cl) | n-Butyl | H | CH | 9.5±0.8 | 19.2±1.4 | 17.4±1.2 | 13.4±1.1 | 8.9±0.7 |
| 5k | C6H4(p-OCH3) | Benzyl | H | CH | 13.7±0.8 | 8.9±0.6 | 16.5±1.3 | 10.6±0.9 | 12.4±0.9 |
| 5l | 3-Pyridyl | n-Butyl | H | CH | 15.3±1.2 | 10.1±0.8 | 26.2±1.7 | 15.3±1.2 | 14.9±1.1 |
| 5m | 2-Thienyl | Benzyl | H | CH | 9.8±0.7 | 9.5±0.9 | 12.2±1.1 | 11.5±0.8 | 16.9±1.2 |
| 5n | H | n-Butyl | H | N | 14.7±1.2 | 7.5±0.5 | 18.6±1.1 | 17.8±1.4 | 23.9±1.7 |
| 5o | H | Benzyl | H | N | 21.2±1.9 | 24.7±2.1 | 32.3±2.5 | 21.1±1.8 | 12.4±1.1 |
| 5p | H | n-Butyl | OCH3 | CH | 14.6±1.3 | 24.3±1.9 | 17.3±1.6 | 27.1±1.9 | 25.7±1.6 |
| 5q | H | n-Butyl | CF3 | CH | 8.7±0.7 | 14.8±1.2 | 12.3±0.9 | 9.4±0.7 | 8.6±0.5 |
| 5r | H | Benzyl | OCH3 | CH | 14.5±1.2 | 6.2±0.4 | 19.1±0.7 | 21.4±1.8 | 33.1±2.3 |
| 5s | H | Benzyl | CF3 | CH | 10.1±0.6 | 14.3±0.8 | 18.3±0.5 | 10.1±0.7 | 4.9±0.3 |
| Cisplatin | 15.8±2.4 | 8.4±0.7 | 4.2±0.7 | 15.4±1.9 | 10.5±2.3 | ||||

| [1] |
(a) https://www.cancer.gov/about-cancer/understanding/statistics;
|
|
(b) http://www.who.int/mediacentre/factsheets/fs297/en.
|
|
| [2] |
Siegel, R. L.; Miller, K. D.; Jemal, A. C. A. Cancer J. Clin. 2017, 67, 7.
doi: 10.3322/caac.21387 |
| [3] |
Yan, L.; Lin, M.; Pan, S.; Assaraf, Y. G.; Wang, Z. W.; Zhu, X. Drug Resistance Updates 2020, 49, 100673.
doi: 10.1016/j.drup.2019.100673 |
| [4] |
Chatterjee, N.; Bivona, T. G. Trends Cancer 2019, 5, 170.
doi: S2405-8033(19)30019-6 pmid: 30898264 |
| [5] |
Gaujac, A.; Navickiene, S.; Collins, M. I.; Brandt, S. D.; Andrade, J. B. Drug Test. Anal. 2012, 4, 636.
doi: 10.1002/dta.1343 pmid: 22577086 |
| [6] |
Xie, Z. J.; Cao, N.; Wang, C. H. Food Chem. 2021, 348, 129067.
doi: 10.1016/j.foodchem.2021.129067 |
| [7] |
Cao, R. H.; Peng, W. L.; Wang, Z. H.; Xu, A. L. Curr. Med. Chem. 2007, 14, 497.
|
| [8] |
Michael, C.; Robert, W. W.; Fil, G.; James, M. C.; Steven, A. B.; Kenner, C. R.; Jacqueline, N. C.; Steven, M. P., Phil, S. J. Med. Chem. 1982, 25, 1081.
doi: 10.1021/jm00351a015 |
| [9] |
Bournine, L.; Bensalem, S.; Fatmi, S.; Bedjou, F.; Mathieu, V.; Iguer-Ouada, M.; Kiss, R.; Duez, P. Eur. J. Integr. Med. 2017, 9, 91.
doi: 10.1016/j.eujim.2016.10.002 |
| [10] |
Sun, Y.; Guo, L.; Fan, W.-X.; Chen, W.; Zhang, J.; Dai, B. Chin. J. Org. Chem. 2021, 41, 400. (in Chinese)
doi: 10.6023/cjoc202006026 |
|
( 孙跃, 郭亮, 范文玺, 陈伟, 张洁, 代斌, 有机化学, 2021, 41, 400.)
doi: 10.6023/cjoc202006026 |
|
| [11] |
Chen, X. F.; Guo, L.; Ma, Q.; Chen, W.; Fan, W. X.; Zhang, J. Molecules 2019, 24, 2950.
doi: 10.3390/molecules24162950 |
| [12] |
Kamboj, A.; Sihag, B.; Brar, D. S.; Kaur, A.; Salunke, D. B. Eur. J. Med. Chem. 2021, 221, 113536.
doi: 10.1016/j.ejmech.2021.113536 |
| [13] |
Srivastava, S. K.; Agarwal, A.; Chauhan, P. M. S.; Agarwal, S. K.; Bhaduri, A. P.; Singh, S. N.; Fatima, N.; Chatterjee, R. K. P. Bioorg. Med. Chem. 1999, 7, 1223.
doi: 10.1016/S0968-0896(99)00050-4 |
| [14] |
Wang, Y. H.; Tang, J. G.; Wang, R. R.; Yang, L. M.; Dong, Z. J.; Du, L.; Shen, X.; Liu, J. K.; Zheng, Y. T. Biochem. Biophys. Res. Commun. 2007, 355, 1091.
doi: 10.1016/j.bbrc.2007.02.081 |
| [15] |
Guo, L.; Xie, J. W.; Fan, W. X.; Chen, W.; Dai, B.; Ma, Q. Chin. J. Org. Chem. 2017, 37, 1741. (in Chinese)
doi: 10.6023/cjoc201701005 |
|
( 郭亮, 谢建伟, 范文玺, 陈伟, 代斌, 马芹, 有机化学, 2017, 37, 1741.)
doi: 10.6023/cjoc201701005 |
|
| [16] |
Sun, Y.; Huo, X.-Y.; Wang, Z.-X.; Han, X.-Q.; Zhang, J. Fine Chem. 2020, 37, 1672. (in Chinese)
|
|
( 孙跃, 霍新玉, 王兆旭, 韩小强, 张洁, 精细化工, 2020, 37, 1672.)
|
|
| [17] |
Huo, X. Y.; Li, W. B.; Zhang, B. Y.; Chen, X. F.; Dai, B. Chin. J. Org. Chem. 2018, 38, 3356. (in Chinese)
doi: 10.6023/cjoc201805053 |
|
( 霍新玉, 李文斌, 张博雅, 陈晓飞, 代斌, 有机化学, 2018, 38, 3356.)
doi: 10.6023/cjoc201805053 |
|
| [18] |
Guo, L.; Chen, W.; Fan, W. X.; Ma, Q.; Sun, R. Q.; Shao, G.; Cao, R. H. Med. Chem. Commun. 2016, 7, 2177.
doi: 10.1039/C6MD00360E |
| [19] |
Huo, X. Y.; Guo, L.; Chen, X. F.; Zhou, Y. T.; Zhang, J.; Han, X. Q.; Dai, B. Molecules 2018, 23, 1344.
doi: 10.3390/molecules23061344 |
| [20] |
Narasimhan, B.; Sharma, D.; Kumar, P. Med Chem Res. 2012, 21, 269.
doi: 10.1007/s00044-010-9533-9 |
| [21] |
Shah, K.; Chhabra, S.; Shrivastava, S. K.; Mishra, P. Med. Chem. Res. 2013, 22, 5077.
doi: 10.1007/s00044-013-0476-9 |
| [22] |
Gaba, M.; Singh, S.; Mohan, C. Eur. J. Med. Chem. 2014, 76, 494.
doi: 10.1016/j.ejmech.2014.01.030 |
| [23] |
Li, N.; Xin, J.-C.; Meng, Y.-Q.; Li, E.-D.; Ma, Q.-S.; Bao, C.-N. Chin. J. Org. Chem. 2018, 38, 368. (in Chinese)
|
|
( 栗娜, 辛景超, 孟娅琪, 李二冬, 马启胜, 包崇男, 有机化学, 2018, 38, 368.)
|
|
| [24] |
Hsieh, C. Y.; Ko, P. W.; Chang, Y. J.; Kapoor, M.; Liang, Y. C.; Chu, H. L. Molecules 2019, 24, 3299.
doi: 10.3390/molecules24183299 |
| [25] |
Abonia, R.; Cortés, E.; Insuasty., B.; Insuasty, B.; Quiroga, J.; Nogueras, M.; Coboet, J. Eur. J. Med. Chem. 2011, 46, 4062.
doi: 10.1016/j.ejmech.2011.06.006 |
| [26] |
Gaba, M.; Singh, S.; Mohan, C. Eur. J. Med. Chem. 2014, 76, 494.
doi: 10.1016/j.ejmech.2014.01.030 |
| [27] |
(a) Sharma, D.; Narasimhan, B.; Kumar, P.; Judge, V.; Narang, R.; Clercq, E. D. J. Enzyme Inhib. Med. Chem. 2009, 24, 1161.
doi: 10.1080/14756360802694427 |
|
(b) Chidambaranathan, V.; Mahalakshmi, C. M. Int. J. Chem. Sci. 2015, 13, 205.
|
|
| [28] |
Karaburun, A. C.; Cavusoglu, K. C.; Cevik, U. A.; Osmaniye, D.; Sağlık, B. N.; Levent, S.; Atlı, O.; Koparal, A. S.; Kaplancıklı, Z. A. Molecules 2019, 24, 191.
doi: 10.3390/molecules24010191 |
| [29] |
Sridevi, C. H.; Balaji, K.; Naidu, A.; Sudhakaran, R. Eur. J. Inorg. Chem. 2012, 7, 234.
|
| [30] |
Chen, Z. Y.; Cao, R. H.; Shi, B. X.; Guo, L.; Sun, J.; Ma, Q.; Fan, W. X.; Song, H. C. Eur. J. Med. Chem. 2011, 46, 5127.
doi: 10.1016/j.ejmech.2011.08.027 |
| [31] |
He, S.; Dobbelaar, P. H.; Guo, L.; Ye, Z.; Jian, L.; Jian, T. Biochem. Biophys. Res. Commun. 2016, 26, 1529.
|
| [32] |
Chen, Q.; Chen, W.; Fan, W. X.; Guo, L.; Ma, Q.; Zhang, X.D.; Du, R. L.; Cao, R. H. Bioorg. Med. Chem. Lett. 2016, 26, 5065.
doi: 10.1016/j.bmcl.2016.08.084 |
| [33] |
Zhao, Y. X.; Wang, Y. Y.; Zhang, C. L.; Xu, X.; Wang, S. F. Chin. J. Org. Chem. 2021, 41, 1224. (in Chinese)
doi: 10.6023/cjoc202009050 |
|
( 赵雨珣, 王芸芸, 张成龙, 徐徐, 王石发, 有机化学, 2021, 41, 1224.)
doi: 10.6023/cjoc202009050 |
| [1] | Haibo Huo, Guixia Li, Shijun Wang, Chun Han, Baojun Shi, Jian Li. Novel γ-Carboline Derivatives as Antibacterial Agents: Synthesis and Antibacterial Evaluation [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 204-215. |
| [2] | Bozhen Wang, Jie Zhang, Chunhui Nian, Mingming Jin, Miaomiao Kong, Wulan Li, Wenfei He, Jianzhang Wu. Synthesis and Antitumor Activity of 3,4-Dichlorophenyl Amides [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 232-241. |
| [3] | Mengjia Xiao, Xike Gao. Design, Synthesis and Anti-inflammatory Activity of Azulene Derivatives Containing Benzimidazole Unit [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3246-3256. |
| [4] | Panxing Pang, Rong Ning, Chuang Zhu, Wenjie Huang, Xianli Ma, Caina Jiang, Fangyao Li, Xiaoqun Zhou. Synthesis and in Vitro Antitumor Activity of Matrine Semicarbazide Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2126-2135. |
| [5] | Xingzhou Liu, Mingjia Yu, Jianhua Liang. Research Progress on the Synthesis of Protoberberine Skeleton and Its Anti-inflammatory Activity [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1325-1340. |
| [6] | Kanghui Duan, Junlong Tang, Wanqing Wu. Recent Advances in the Synthesis of Fused Heterocyclic Compounds and Their Antitumor Activities [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 826-854. |
| [7] | Chujie Liao, Hongyao Ruan, Junfeng Jiang, Lun Luo, Yanggen Hu. Synthesis and Activity Evaluation of 3-Aryl-2-imino-benzo[e][1,3]-oxazin-4-ol Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 763-770. |
| [8] | Weiqin Liu, Lihui Shao, Chengpeng Li, Yayu Zou, Haitao Long, Yan Li, Qiangsheng Ge, Zhenchao Wang, Guiping Ouyang. Synthesis and Antitumor Activity of 3-Hydrazone Quinazolinone Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 214-222. |
| [9] | Guangping Liang, Wei Wang, Xuxiu Zhu, Guangyan Liang, Jun Yang, Daoping Wang. Synthesis and in Vitro Anti-tumor Activity of Novel Spliced Compounds of Zidovudine and 4-Anilinoquinazolines [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2793-2805. |
| [10] | Rong Zhang, Xiang Gao, Lingling Chen, Fajun Nan. Discovery and Structure-Activity Relationship Studies of Thiazole- Oxazole Tandem Heterocyclic RNA Splicing Inhibitors [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2925-2939. |
| [11] | Binghan Lin, Jibin Zhuo, Caixia Lin, Yong Gao, Yaofeng Yuan. Synthesis and Nucleotide Recognition Properties of Carborane-Based Benzoimidazolium Cyclophane [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2551-2558. |
| [12] | Jing Zhao, Zhe Jin, Run Wang, Xin'geng Zhang, Yingmei Han, Chun Hu, Xiaoping Liu, Chuanming Zhang, Liping Jin. Design, Synthesis and Anticancer Activity of 2-((Pyridin- 2-ylmethyl)thio)-1H-benzimidazole Derivatives [J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2172-2183. |
| [13] | Dongyan Hu, Guangtian Han, Xi'an Li, Huazhong Ren, Lirong Yue, Li Guo, Jiafu Feng. Synthesis and Evaluation in vitro of Novel Harmine Derivatives as Anticancer Activity Agents [J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1863-1871. |
| [14] | Lu Xue, Lihua Zhang, Chengyu Zhang, Xin Zhao, Weifan Dang, Zhaoxin Wang, Chunhua Wang, Tongchuan Suo, Xiaohui Yan. Discovery of Tiancimycin Congeners from Streptomyces sp. CB03234-S [J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 1241-1247. |
| [15] | Yan Zeng, Lifei Nie, Chao Niu, Aytilla Mamatjan, Khurshed Bozorov, Jiangyu Zhao, Haji Akber Aisa. Synthesis and Biological Activities of Dihydrooxazolo[5,4-d]-pyrrolo[1,2-a]pyrimidinones [J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 543-556. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||