Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (11): 3730-3739.DOI: 10.6023/cjoc202205026 Previous Articles Next Articles
ARTICLES
魏兆鑫, 王仁杰, 张永红, 王斌, 夏昱, 金伟伟*( ), 刘晨江*(
), 刘晨江*( )
)
收稿日期:2022-05-18
修回日期:2022-07-01
发布日期:2022-07-20
通讯作者:
金伟伟, 刘晨江
基金资助:
Zhaoxin Wei, Renjie Wang, Yonghong Zhang, Bin Wang, Yu Xia, Weiwei Jin( ), Chenjiang Liu(
), Chenjiang Liu( )
)
Received:2022-05-18
Revised:2022-07-01
Published:2022-07-20
Contact:
Weiwei Jin, Chenjiang Liu
Supported by:Share
Zhaoxin Wei, Renjie Wang, Yonghong Zhang, Bin Wang, Yu Xia, Weiwei Jin, Chenjiang Liu. Electrochemical Synthesis of N-Acyl/Sulfonylsulfenamides Using Potassium Iodide as Mediator[J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3730-3739.
| Entry | Variation from the standard conditions | Yieldb/% |
|---|---|---|
| 1 | None | 98 |
| 2 | KBr instead of KI | 90 |
| 3 | KCl instead of KI | 13 |
| 4 | NaBr instead of KI | 87 |
| 5 | NaI instead of KI | 88 |
| 6 | TBAI instead of KI | 48 |
| 7 | nBu4NBF4 instead of KI | n.d. |
| 8 | 15-Crown-5 instead of 18-crown-6 | 50 |
| 9 | Dibenzo-18-crown-6 instead of 18-crown-6 | 46 |
| 10 | MeOH instead of CH2Cl2 | 21 |
| 11 | MeCN instead of CH2Cl2 | 69 |
| 12 | DMF instead of CH2Cl2 | 70 |
| 13 | Pt(+)/Pt(-) instead of Pt(+)/Ni(-) | 94 |
| 14 | C(+)/Ni(-) instead of Pt(+)/Ni(-) | 72 |
| 15 | Without 18-crown-6 | n.d. |
| 16 | Without KI | n.d. |
| 17 | Without electric current | n.d. |
| Entry | Variation from the standard conditions | Yieldb/% |
|---|---|---|
| 1 | None | 98 |
| 2 | KBr instead of KI | 90 |
| 3 | KCl instead of KI | 13 |
| 4 | NaBr instead of KI | 87 |
| 5 | NaI instead of KI | 88 |
| 6 | TBAI instead of KI | 48 |
| 7 | nBu4NBF4 instead of KI | n.d. |
| 8 | 15-Crown-5 instead of 18-crown-6 | 50 |
| 9 | Dibenzo-18-crown-6 instead of 18-crown-6 | 46 |
| 10 | MeOH instead of CH2Cl2 | 21 |
| 11 | MeCN instead of CH2Cl2 | 69 |
| 12 | DMF instead of CH2Cl2 | 70 |
| 13 | Pt(+)/Pt(-) instead of Pt(+)/Ni(-) | 94 |
| 14 | C(+)/Ni(-) instead of Pt(+)/Ni(-) | 72 |
| 15 | Without 18-crown-6 | n.d. |
| 16 | Without KI | n.d. |
| 17 | Without electric current | n.d. |
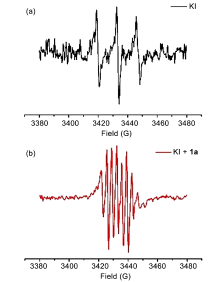

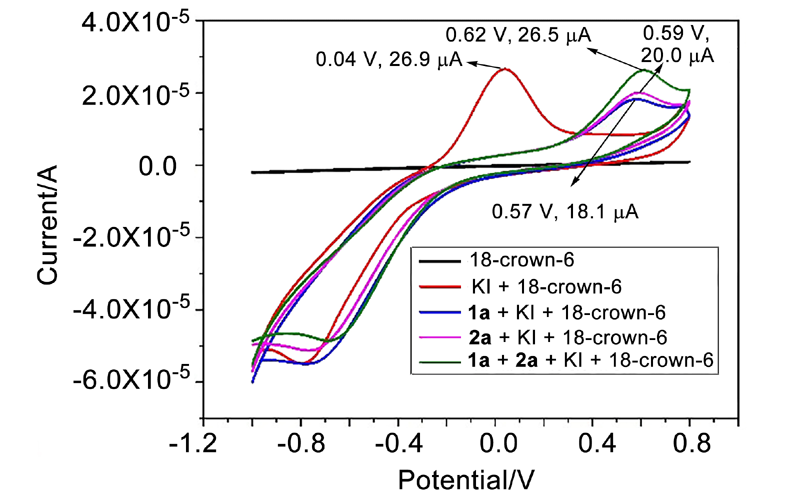
| [1] |
(a) Bowman, W. R.; Clark, D. N.; Marmon, R. J. Tetrahedron 1994, 50, 1275.
doi: 10.1016/S0040-4020(01)80837-1 pmid: 17604170 |
|
(b) Matsuo, J. I.; Iida, D.; Yamanaka, H.; Mukaiyama, T. Tetrahedron 2003, 59, 6739.
doi: 10.1016/S0040-4020(03)00479-4 pmid: 17604170 |
|
|
(c) Heldreth, B.; Long, T. E.; Jang, S.; Reddy, G.; Turos, E.; Dickey, S.; Lim, D. V. Bioorg. Med. Chem. 2006, 14, 3775.
pmid: 17604170 |
|
|
(d) Guarino, V. R.; Karunaratne, V.; Stella, V. J. Bioorg. Med. Chem. Lett. 2007, 17, 4910.
pmid: 17604170 |
|
|
(e) Aota, Y.; Kano, T.; Maruoka, K. Angew. Chem., Int. Ed. 2019, 58, 17661.
doi: 10.1002/anie.201911021 pmid: 17604170 |
|
| [2] |
Craine, L.; Raban, M. Chem. Rev. 1989, 89, 689.
doi: 10.1021/cr00094a001 |
| [3] |
Steinkamp, A. D.; Schmitt, L.; Chen, X.; Fietkau, K.; Heise, R.; Baron, J. M.; Bolm, C. Skin Pharmacol. Phys. 2016, 29, 281.
|
| [4] |
Guarino, V. R.; Olson, R. E.; Everlof, J. G.; Wang, N.; McDonald, I.; Haskell, R.; Clarke, W.; Lentz, K. A. Bioorg. Med. Chem. Lett. 2020, 30, 126856.
doi: 10.1016/j.bmcl.2019.126856 |
| [5] |
Petkowski, J. J.; Bains, W.; Seager, S. Astrobiology 2018, 19, 579.
doi: 10.1089/ast.2018.1831 |
| [6] |
(a) Barton, D. H. R.; Hesse, R. H.; O'Sullivan, A. C.; Pechet, M. M. J. Org. Chem. 1991, 56, 6702.
doi: 10.1021/jo00023a040 |
|
(b) Bao, M.; Shimizu, M.; Shimada, S.; Tanaka, M. Tetrahedron 2003, 59, 303.
doi: 10.1016/S0040-4020(02)01554-5 |
|
| [7] |
Zhang, X.-S.; Zhang, X.-H. Phosphorus, Sulfur Silicon Relat. Elem. 2016, 191, 89.
doi: 10.1080/10426507.2015.1012670 |
| [8] |
Lee, C.; Lim, Y. N.; Jang, H.-Y. Eur. J. Org. Chem. 2015, 5934.
|
| [9] |
(a) Torii, S.; Tanaka, H.; Ukida, M. J. Org. Chem. 1979, 44, 1554.
doi: 10.1021/jo01323a039 |
|
(b) Li, C.-J. Acc. Chem. Res. 2009, 42, 335.
doi: 10.1021/ar800164n |
|
|
(c) Girard, S. A..; Knauber, T..; Li, C.-J. Angew. Chem., Int. Ed. 2014, 53, 74.
|
|
|
(d) Wang, H.; Zhang, J.; Tan, J.; Xin, L.; Li, Y.; Zhang, S.; Xu, K. Org. Lett. 2018, 20, 2505.
doi: 10.1021/acs.orglett.8b00165 |
|
|
(e) Mo, Z.-Y.; Swaroop, T. R.; Tong, W.; Zhang, Y.-Z.; Tang, H.-T.; Pan, Y.-M.; Sun, H.-B.; Chen, Z.-F. Green Chem. 2018, 20, 4428.
doi: 10.1039/C8GC02143K |
|
|
(f) Deng, L.; Wang, Y.; Mei, H.; Pan, Y.; Han, J. J. Org. Chem. 2019, 84, 949.
doi: 10.1021/acs.joc.8b02882 |
|
|
(g) Li, Y.; Yang, Q.; Yang, L.; Lei, N.; Zheng, K. Chem. Commun. 2019, 55, 4981.
doi: 10.1039/C9CC01378D |
|
|
(h) Tang, S.; Liu, Y.; Li, L.; Ren, X.; Li, J.; Yang, G.; Li, H.; Yuan, B. Org. Biomol. Chem. 2019, 17, 1370.
doi: 10.1039/C8OB03211D |
|
|
(i) Meng, Z.-Y.; Feng, C.-T.; Zhang, L.; Yang, Q.; Chen, D.-X.; Xu, K. Org. Lett. 2021, 23, 4214.
doi: 10.1021/acs.orglett.1c01161 |
|
|
(j) Deng, Y.; You, S.; Ruan, M.; Wang, Y.; Chen, Z.; Yang, G.; Gao, M. Adv. Synth. Catal. 2021, 363, 464.
doi: 10.1002/adsc.202000997 |
|
|
(k) Tian, Z.; Gong, Q.; Huang, T.; Liu, L.; Chen, T. J. Org. Chem. 2021, 86, 15914.
doi: 10.1021/acs.joc.1c00260 |
|
|
(l) Du, Z.; Qi, Q.; Gao, W.; Ma, L.; Liu, Z.; Wang, R.; Chen, J. Chem. Rec. 2021, 22, e202100178.
|
|
|
(m) Li, J.; Zhang, S.; Xu, K. Chin. Chem. Lett. 2021, 32, 2729.
doi: 10.1016/j.cclet.2021.03.027 |
|
| [10] |
(a) Li, W.-C.; Zeng, C.-C.; Hu, L.-M.; Tian, H.-Y.; Little, R. D. Adv. Synth. Catal. 2013, 355, 2884.
doi: 10.1002/adsc.201300502 |
|
(b) Liang, S.; Zeng, C.-C.; Tian, H.-Y.; Sun, B.-G.; Luo, X.-G.; Ren, F.-Z. Adv. Synth. Catal. 2018, 360, 1444.
doi: 10.1002/adsc.201701401 |
|
|
(c) Zhang, H. H.; Wang, Y. Q.; Huang, L. T.; Zhu, L. Q.; Feng, Y. Y.; Lu, Y. M.; Zhao, Q. Y.; Wang, X. Q.; Wang, Z. Chem. Commun. 2018, 54, 8265.
doi: 10.1039/C8CC04471F |
|
|
(d) Sun, C.-C.; Lian, F.; Xu, K.; Zeng, C.-C.; Sun, B.-G. Adv. Synth. Catal. 2019, 361, 4041.
doi: 10.1002/adsc.201900537 |
|
|
(e) Zhang, S.; Li, L.; Xue, M.; Zhang, R.; Xu, K.; Zeng, C. Org. Lett. 2018, 20, 3443.
doi: 10.1021/acs.orglett.8b00981 |
|
|
(f) Li, Y.; Sun, C.-C.; Zeng, C.-C. J. Electroanal. Chem. 2020, 861, 113941.
doi: 10.1016/j.jelechem.2020.113941 |
|
|
(g) Huynh, T. N. T.; Tankam, T.; Koguchi, S.; Rerkrachaneekorn, T.; Sukwattanasinitt, M.; Wacharasindhu, S. Green Chem. 2021, 23, 5189.
doi: 10.1039/D1GC01131F |
|
|
(h) Lian, F.; Xu, K.; Zeng, C. Chem. Rec. 2021, 21, 2290.
doi: 10.1002/tcr.202100036 |
|
| [11] |
Sawamura, T.; Takahashi, K.; Inagi, S.; Fuchigami, T. Angew. Chem., Int. Ed. 2012, 51, 4413.
doi: 10.1002/anie.201200438 |
| [12] |
Wei, Z.; Wang, R.; Zhang, Y.; Wang, B.; Xia, Y.; Abdukader, A.; Xue, F.; Jin, W.; Liu, C. Eur. J. Org. Chem. 2021, 4728.
|
| [13] |
(a) Dong, X.; Wang, R.; Jin, W.; Liu, C. Org. Lett. 2020, 22, 3062.
doi: 10.1021/acs.orglett.0c00814 |
|
(b) Wang, R.; Dong, X.; Zhang, Y.; Wang, B.; Xia, Y.; Abdukader, A.; Xue, F.; Jin, W.; Liu, C. Chem.-Eur. J. 2021, 27, 14931.
doi: 10.1002/chem.202102262 |
|
|
(c) Cheng, Z.; Gao, X.; Yao, L.; Wei, Z.; Qin, G.; Zhang, Y.; Wang, B.; Xia, Y.; Abdukader, A.; Xue, F.; Jin, W.; Liu, C. Eur. J. Org. Chem. 2021, 3743.
|
|
| [14] |
(a) Jin, W.; Zheng, P.; Wong, W.-T.; Law, G.-L. Adv. Synth. Catal. 2017, 359, 1588.
doi: 10.1002/adsc.201601065 pmid: 31675240 |
|
(b) Cheng, Z.; Jin, W.; Liu, C. Org. Chem. Front. 2019, 6, 841.
doi: 10.1039/c8qo01412d pmid: 31675240 |
|
|
(c) Cheng, Z.; Sun, P.; Tang, A.; Jin, W.; Liu, C. Org. Lett. 2019, 21, 8925.
doi: 10.1021/acs.orglett.9b03192 pmid: 31675240 |
|
|
(d) Chen, Z.; Jin, W.; Xia, Y.; Zhang, Y.; Xie, M.; Ma, S.; Liu, C. Org. Lett. 2020, 22, 8261.
doi: 10.1021/acs.orglett.0c02907 pmid: 31675240 |
|
| [15] |
Kost, D.; Zeichner, A. Tetrahedron Lett. 1975, 16, 3239.
doi: 10.1016/S0040-4039(00)91466-7 |
| [1] | Wei Meng, Kun Xu, Bingbing Guo, Chengchu Zeng. Recent Advances in Minisci Reactions under Electrochemical Conditions [J]. Chinese Journal of Organic Chemistry, 2021, 41(7): 2621-2635. |
| [2] | Muxue He, Shiyan Cheng, Yongzhou Pan, Haitao Tang, Yingming Pan. Electrochemically Mediated S—N Bond Formation: Synthesis of Sulfenamides [J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2354-2360. |
| [3] | Shiyan Cheng, Chuhong Ou, Hongmin Lin, Junsong Jia, Haitao Tang, Yingming Pan, Guobao Huang, Xiujin Meng. Electrochemically Mediated Esterification of Aromatic Aldehydes with Aliphatic Alcohols via Anodic Oxidation [J]. Chinese Journal of Organic Chemistry, 2021, 41(12): 4718-4724. |
| [4] | Hehua Xu, Xiangtai Meng, Yu Zheng, Jinyue Luo, Shenlin Huang. Electrochemical Annulations of o-Alkynylanilines for Synthesis of 3-Iodoindoles [J]. Chinese Journal of Organic Chemistry, 2021, 41(12): 4696-4703. |
| [5] | Pan Chao, Liu Peng, Wu An'guo, Li Ming, Wen Lirong, Guo Weisi. Electrochemical-Promoted Synthesis of 2-Thiazolines via Selenylation/Cyclization of N-Allylthioamides [J]. Chinese Journal of Organic Chemistry, 2020, 40(9): 2855-2862. |
| [6] | Li Mengfan, Wang Rong, Hao Wenjuan, Jiang Bo. Electrocatalytic Synthesis of 2,5-Disubstituted 1,3,4-Oxadiazoles [J]. Chinese Journal of Organic Chemistry, 2020, 40(6): 1540-1548. |
| [7] | Feng Enqi, Hou Zhongwei, Xu Haichao. Electrochemical Synthesis of Tetrasubstituted Hydrazines by Dehydrogenative N-N Bond Formation [J]. Chin. J. Org. Chem., 2019, 39(5): 1424-1428. |
| [8] | BIAN Yan-Jiang, ZHANG Gao-Feng. Reductive Coupling Reactions of Aromatic Aldehydes Using Zinc as Electrode in Aqueous Media [J]. Chin. J. Org. Chem., 2010, 30(08): 1237-1239. |
| [9] | LIN, Mei-Yu; WANG, Huan; ZHANG, Ai-Jian ; ZHANG, Gui-Rong ; LU, Jia-Xing*. Electrocarboxylation of Benzalacetophenone: Synthesis of 2,4-Diphenyl-4-oxobutanoic acid [J]. Chin. J. Org. Chem., 2008, 28(9): 1572-1577. |
| [10] | NIU Dong-Fang,LUO Yi-Wen,ZHANG Li,XIAO Li-Ping,LU Jia-Xing*. Electrosynthesis of Dimethyl Carbonate from CO2 in Mild Condition [J]. Chin. J. Org. Chem., 2008, 28(05): 832-836. |
| [11] | BIAN Yan-Jiang*,1,YANG Wei-Min,LI Ji-Tai2. Studies on the Electrosynthesis of Pinacols [J]. Chin. J. Org. Chem., 2006, 26(08): 1130-1132. |
| [12] | ZHOU Xing-Fu, ZHAO Jun-Feng, HE Hui, CHU Dao-Bao. Direct Electrochemical Preparation of Copper Complexes [J]. Chinese Journal of Organic Chemistry, 2004, 24(1): 106-108. |
| [13] | Zhao Ping;Yin Yingwu. Application of Electroauxiliaries in Electroorganic Synthesis [J]. Chin. J. Org. Chem., 2003, 23(7): 628-635. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||