-
-
 About the Cover:Hypervalent iodine reagents have been widely applied in various types of organic transformation including oxida-tive coupling, functionalization, and rearrangement reac-tions. The oxidative rearrangement reactions can be classified into many types including C to X migration (X=C, N or O), X to C migration (X=N, O, S, Si or I), I to X migration (X=O or N) and sigmatropic rear-rangement. The oxidative rearrangement reactions enabled by hypervalent iodine reagents are comprehensively summarized by Yang, Cheng, Zhang, Dong, Han and Du on page 3456.
About the Cover:Hypervalent iodine reagents have been widely applied in various types of organic transformation including oxida-tive coupling, functionalization, and rearrangement reac-tions. The oxidative rearrangement reactions can be classified into many types including C to X migration (X=C, N or O), X to C migration (X=N, O, S, Si or I), I to X migration (X=O or N) and sigmatropic rear-rangement. The oxidative rearrangement reactions enabled by hypervalent iodine reagents are comprehensively summarized by Yang, Cheng, Zhang, Dong, Han and Du on page 3456. -
 About the Cover:A [1+4] annulation of 1,2-dicarbonyl compounds with α,β-unsaturated ketones has been realized under the me- diation of P(NMe2)3 by Xue, Zeng, Yan, Han and He on page 3805. The reaction readily produces polysub?stituted 2,3-dihydrofurans bearing a quaternary carbon center in fair to good yields with a broad substrate scope. Mechanism and chemoselectivity are discussed with the aid of density functional theory (DFT) calcu?lation calculation.
About the Cover:A [1+4] annulation of 1,2-dicarbonyl compounds with α,β-unsaturated ketones has been realized under the me- diation of P(NMe2)3 by Xue, Zeng, Yan, Han and He on page 3805. The reaction readily produces polysub?stituted 2,3-dihydrofurans bearing a quaternary carbon center in fair to good yields with a broad substrate scope. Mechanism and chemoselectivity are discussed with the aid of density functional theory (DFT) calcu?lation calculation. -
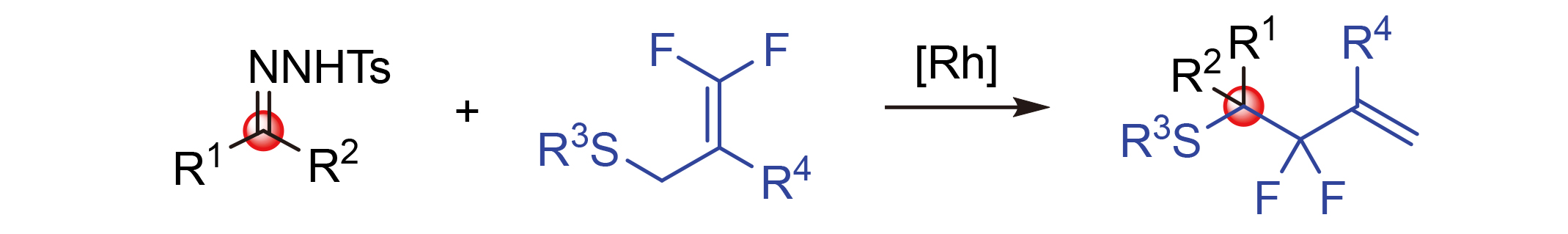
 About the Cover:A rhodium-catalyzed rearrangement reaction of N-tosyl-hydrazones and 3,3-difluoroallyl sulfides was developed by Wang, Teng, Xiong, Xiao and Jiang on page 3658. This protocal provided a facile and efficient synthesis route to gem-difluoroallyl compounds with medium to excellent yield and good functional group tolerance. Moreover, the applicability of current method has been demonstrated by one-pot reaction, gram-scale synthesis and further transformations.
About the Cover:A rhodium-catalyzed rearrangement reaction of N-tosyl-hydrazones and 3,3-difluoroallyl sulfides was developed by Wang, Teng, Xiong, Xiao and Jiang on page 3658. This protocal provided a facile and efficient synthesis route to gem-difluoroallyl compounds with medium to excellent yield and good functional group tolerance. Moreover, the applicability of current method has been demonstrated by one-pot reaction, gram-scale synthesis and further transformations.
-
-
Current Issue
REVIEWS ARTICLES Original article ARTICLES ARTICLES NOTES HIGHLIGHTS
REVIEWS
ARTICLES
Original article
ARTICLES
ARTICLES
NOTES
HIGHLIGHTS